on AMOEBA (EPA:ALMIB)
Amoéba progresses towards the marketing of its biocontrol solution in Europe
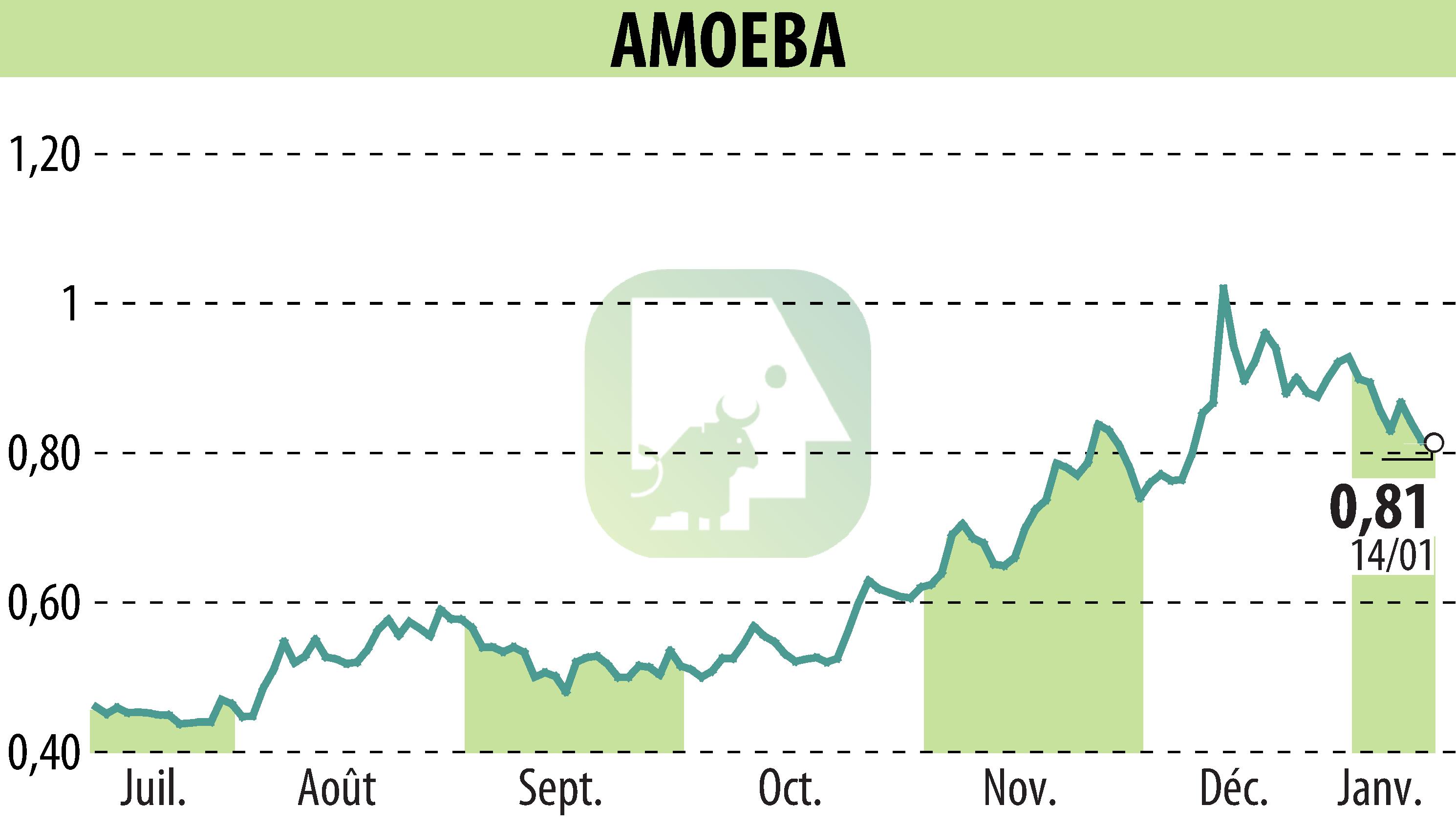
Amoéba announced that the European Food Safety Authority (EFSA) has published the final report on their biocontrol active substance, confirming its fungicidal efficacy and low-risk profile. This validation allows Amoéba to begin the authorisation process for its product “AXPERA” in eight European countries, with a decision expected by the end of 2025.
This crucial step paves the way for commercialisation in Europe, while US federal approval is still pending, with a verdict expected in mid-2025. The European Commission must now confirm the approval within six months of this EFSA publication.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all AMOEBA news
