on Aspire Biopharma, Inc. (NASDAQ:PWUP)
Aspire Biopharma Completes Dosing in Phase 1 Trial of High-Dose Aspirin
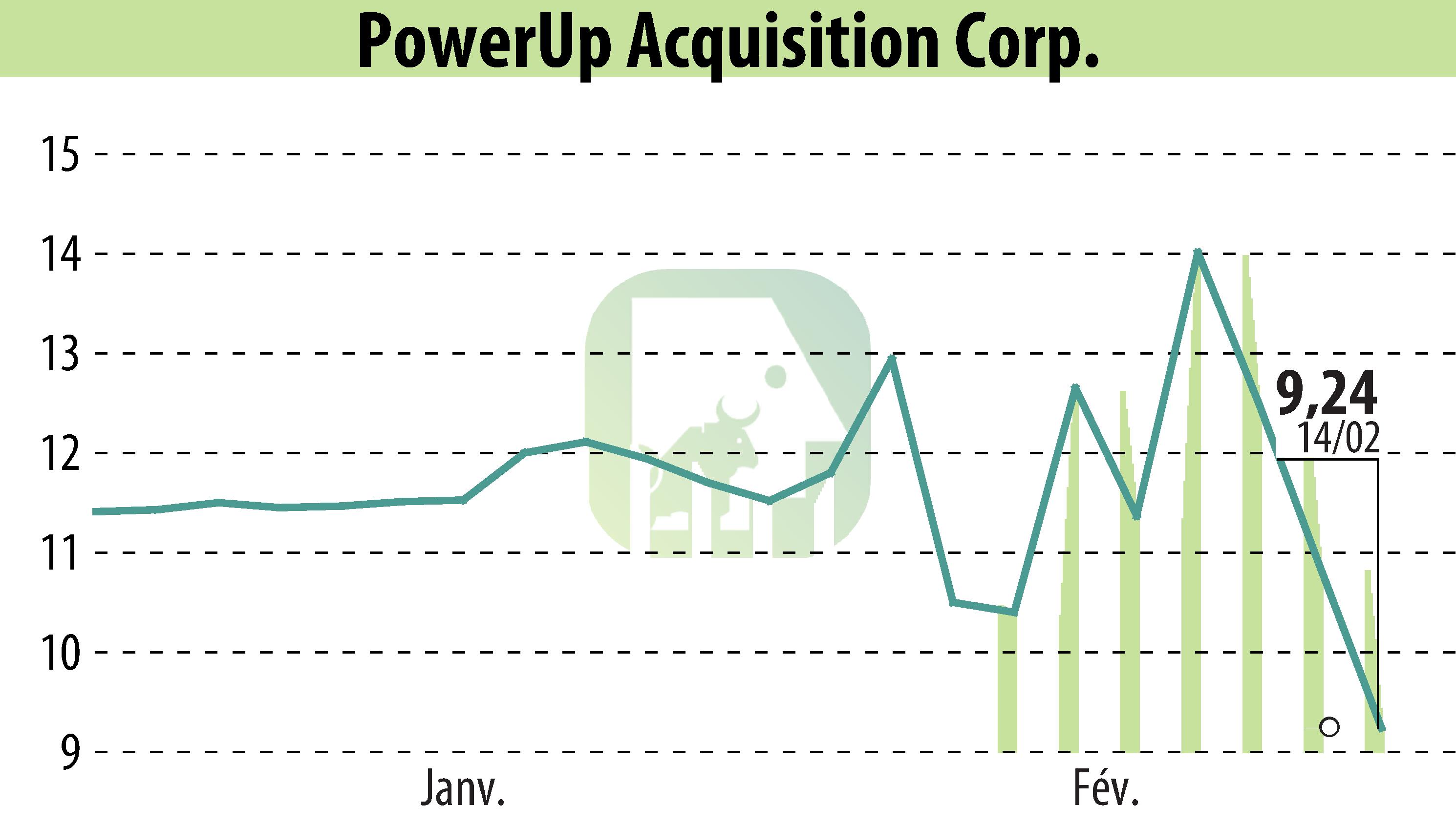
Aspire Biopharma Holdings, Inc. has announced the completion of patient dosing in its Phase 1 clinical trial for an oral transmucosal fast-acting high-dose aspirin formulation. The company expects topline results by mid-Q3 2025. CEO Michael Howe expressed gratitude towards participants and highlighted the milestone as a testament to their team's operational efficiency.
The trial compares the pharmacokinetic and pharmacodynamic characteristics of high-dose aspirin administered sublingually versus standard oral aspirin in healthy adults. Primary outcomes focus on plasma aspirin concentration and platelet aggregation metrics, key indicators of the formulation's rapid impact on cardiac events.
Aspire plans to seek FDA approval post-trial and integrate findings into its development strategy, with an eye on partnership opportunities. The study is crucial for their targeted oral delivery platform that enhances rapid drug absorption.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Aspire Biopharma, Inc. news
