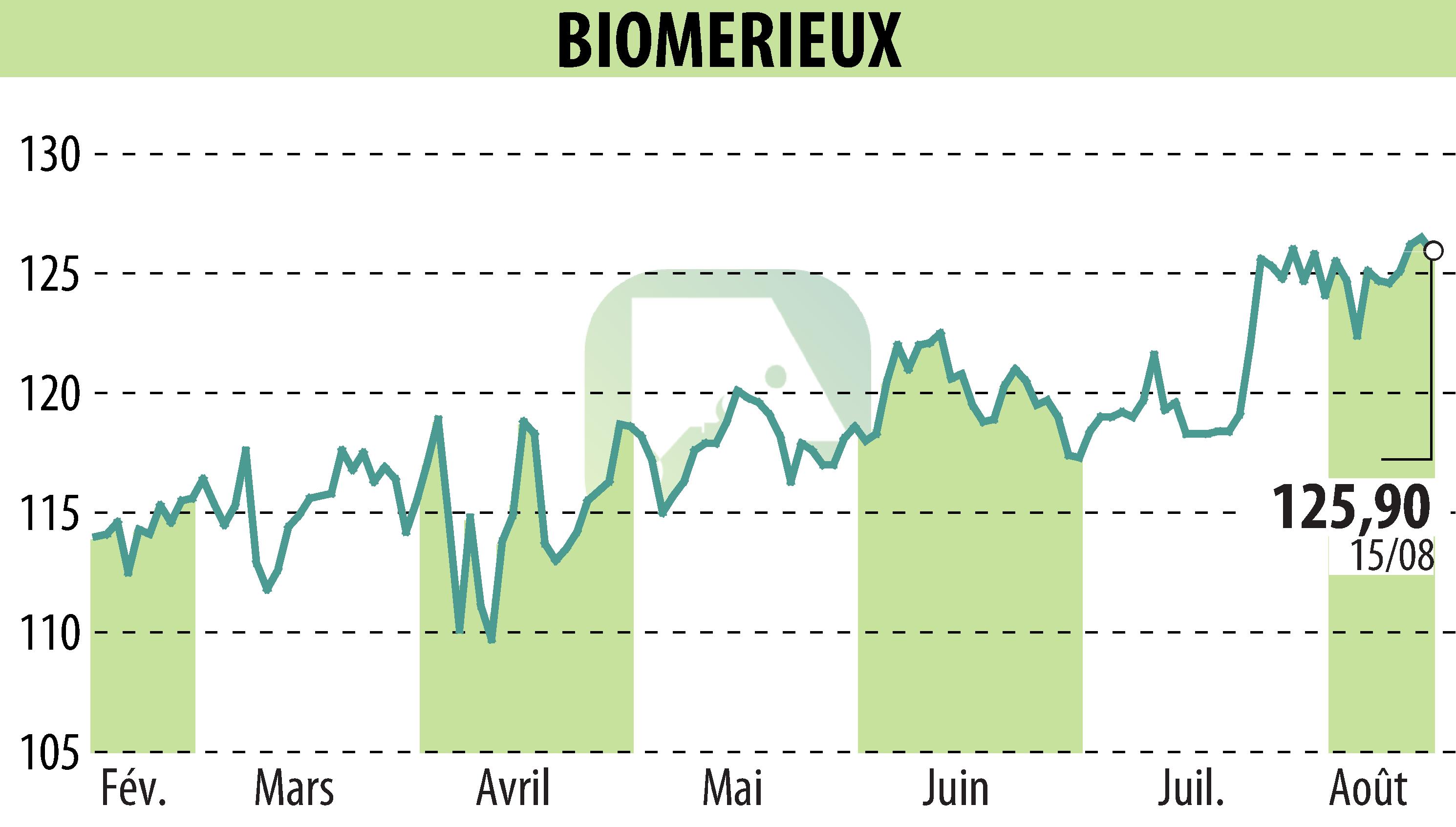
on BIOMERIEUX (EPA:BIM)
BioMérieux Gains U.S. Clearance for Anterior Nasal Swab on Diagnostic Panel
bioMérieux, a leader in in vitro diagnostics, has received U.S. FDA 510(k) clearance and a CLIA waiver for its BIOFIRE® SPOTFIRE® Respiratory/Sore Throat Panel Mini, now including Anterior Nasal Swab (ANS) specimens. This development intends to improve patient comfort by utilizing a non-invasive sampling method that maintains reliability.
The COVID-19 pandemic has heightened the importance of Point-of-Care testing. ANS, favored for ease and comfort, aligns with the trend towards decentralizing diagnostics by offering quick detection of respiratory infections. The BIOFIRE® SPOTFIRE® R/ST Panel Mini, launched in 2024, detects major viral and bacterial infections in 15 minutes.
This advancement reflects bioMérieux's commitment to enhancing patient experiences and maintaining a strong presence in the Point-of-Care market. The ANS specimen will be available in the U.S. by the third quarter of 2025.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all BIOMERIEUX news
