on EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical Reveals Promising Interim Results from VenoValve Study
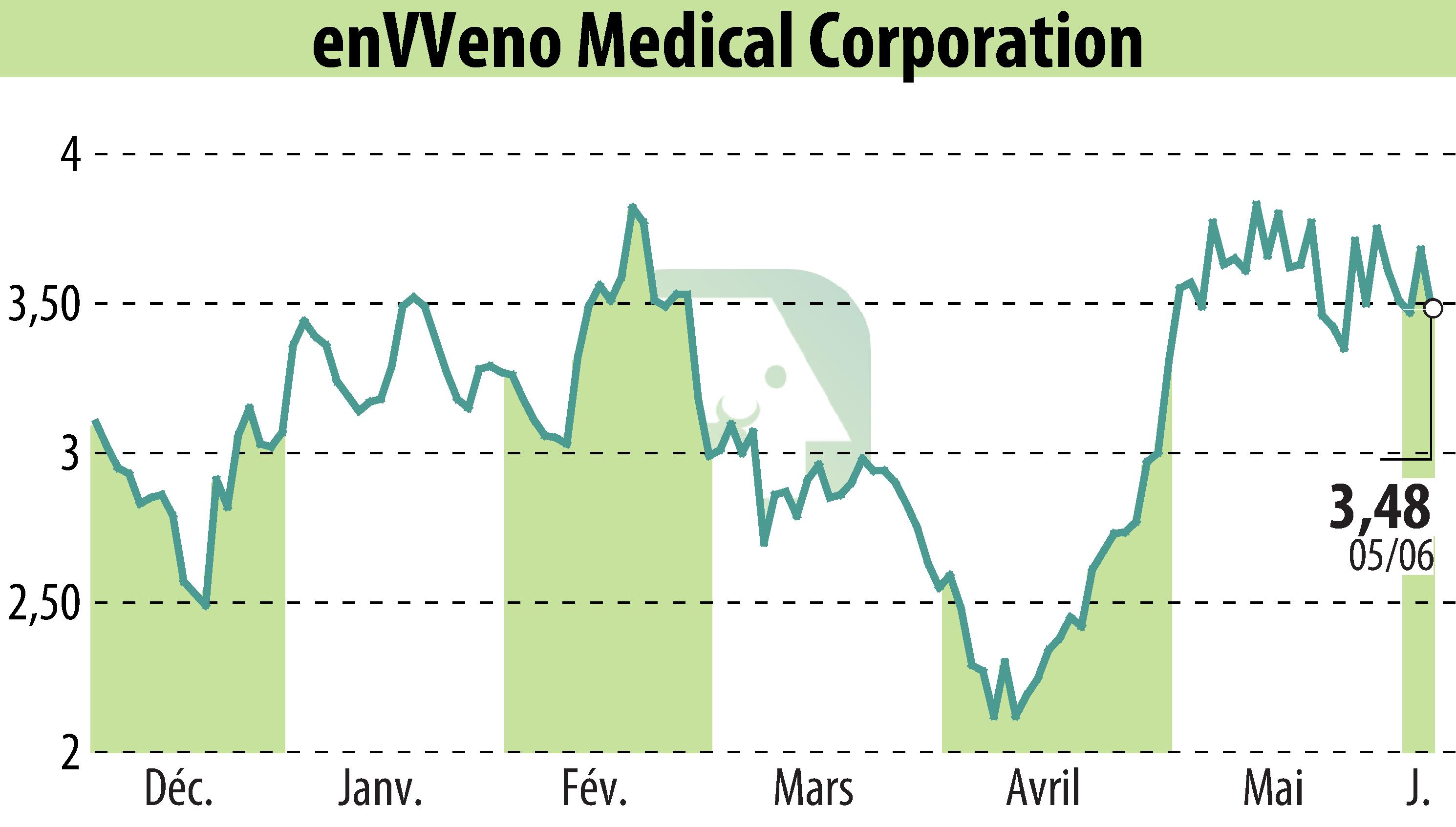
EnVVeno Medical Corporation has announced positive interim two-year data from its VenoValve pivotal trial. Out of 42 participants, 83% maintained a clinically significant improvement in the Revised Venous Clinical Severity Score (rVCSS), with an average enhancement of 9.1 points within the responsive cohort. Participants also reported a median 74% decrease in leg pain, as measured by the Visual Analog Scale. Additional patient-reported outcomes highlighted sustained enhancements across all venous-specific quality-of-life measures.
The data were presented at the Society for Vascular Surgery’s 2025 Vascular Annual Meeting in New Orleans. The company awaits a decision from the FDA on its pre-market authorization application for the VenoValve in the latter half of 2025. The VenoValve is positioned as a potential breakthrough in treating severe chronic venous insufficiency (CVI) caused by malfunctioning deep vein valves.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all EnVVeno Medical Corporation news
