on Nanohale AG (ETR:FYB)
FDA Approves Nufymco® as an Interchangeable Ranibizumab Biosimilar
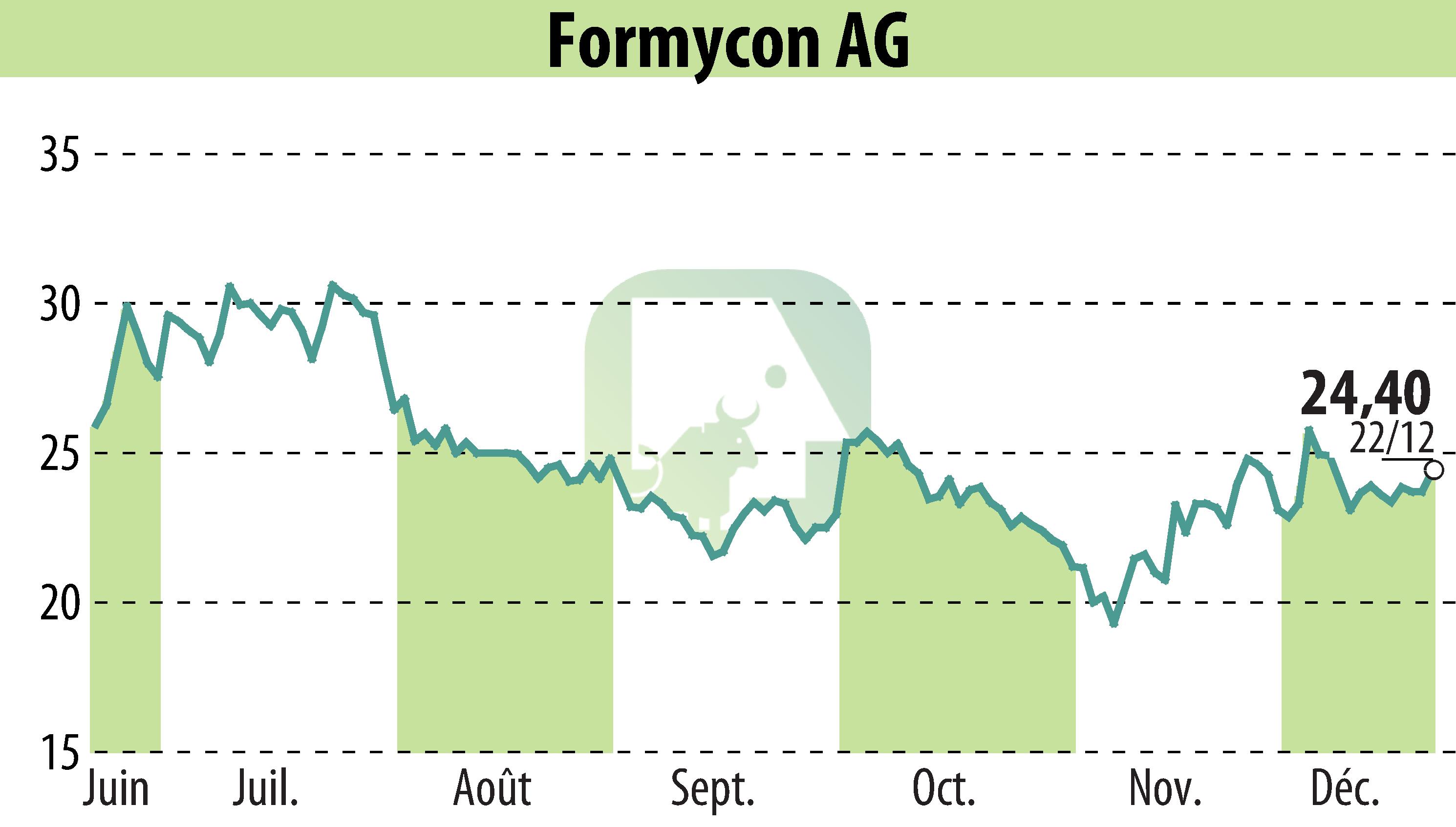
The U.S. FDA has granted approval for Formycon AG's new interchangeable ranibizumab biosimilar, Nufymco®, marking a significant progress in biosimilar development. This achievement reinforces Formycon's pioneering status in the sector with Bioeq AG as its partner.
Formycon has joined forces with Zydus Lifesciences Limited as their commercialization partner in the US, aiming to broaden market access. Nufymco® will be available for various eye conditions, providing a cost-effective alternative for patients.
The partnership with Zydus bolsters Formycon's strategy for greater market penetration, leveraging Zydus' expertise in the US regulatory landscape. This development underscores the importance of strategic alliances in enhancing access to affordable biopharmaceutical therapies.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
