on GENSIGHT BIOLOGICS S.A. (EPA:SIGHT)
GenSight Biologics Receives Compassionate Use Authorization for GS010/LUMEVOQ® in France
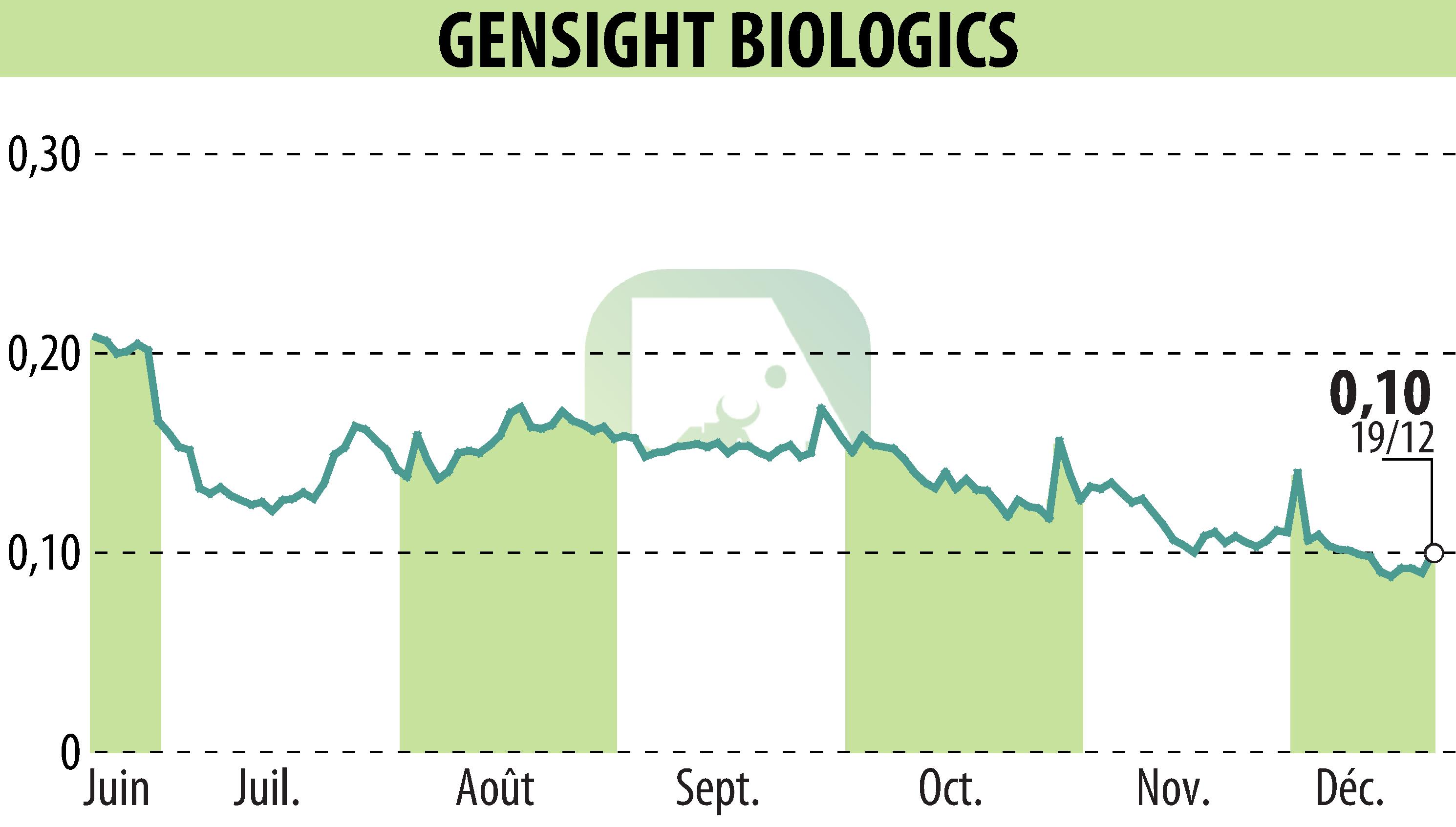
GenSight Biologics, a clinical-stage biopharma company, has announced the approval of a compassionate use authorization for their gene therapy GS010/LUMEVOQ® by the French medicines agency ANSM. This authorization allows early access for patients suffering from ND4-LHON, a rare genetic disease affecting vision.
The compassionate use program in France is designed for patients with serious, rare, or disabling diseases, providing them with treatments that lack marketing authorization, addressing unmet medical needs. The program requires a favorable benefit-risk assessment and is initiated by healthcare professionals who submit individual requests. Eligible patients must meet specific criteria related to the duration of their vision loss.
Leber Hereditary Optic Neuropathy (LHON) is a rare genetic condition causing retinal degeneration and vision loss. The ND4 mutation leads to the most severe outcomes among its types. GS010/LUMEVOQ® leverages proprietary mitochondrial targeting technology for treatment.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all GENSIGHT BIOLOGICS S.A. news
