on Heidelberg Pharma AG (ETR:HPHA)
FDA Requests Additional Data from Telix Pharmaceuticals, Delaying Payment to Heidelberg Pharma
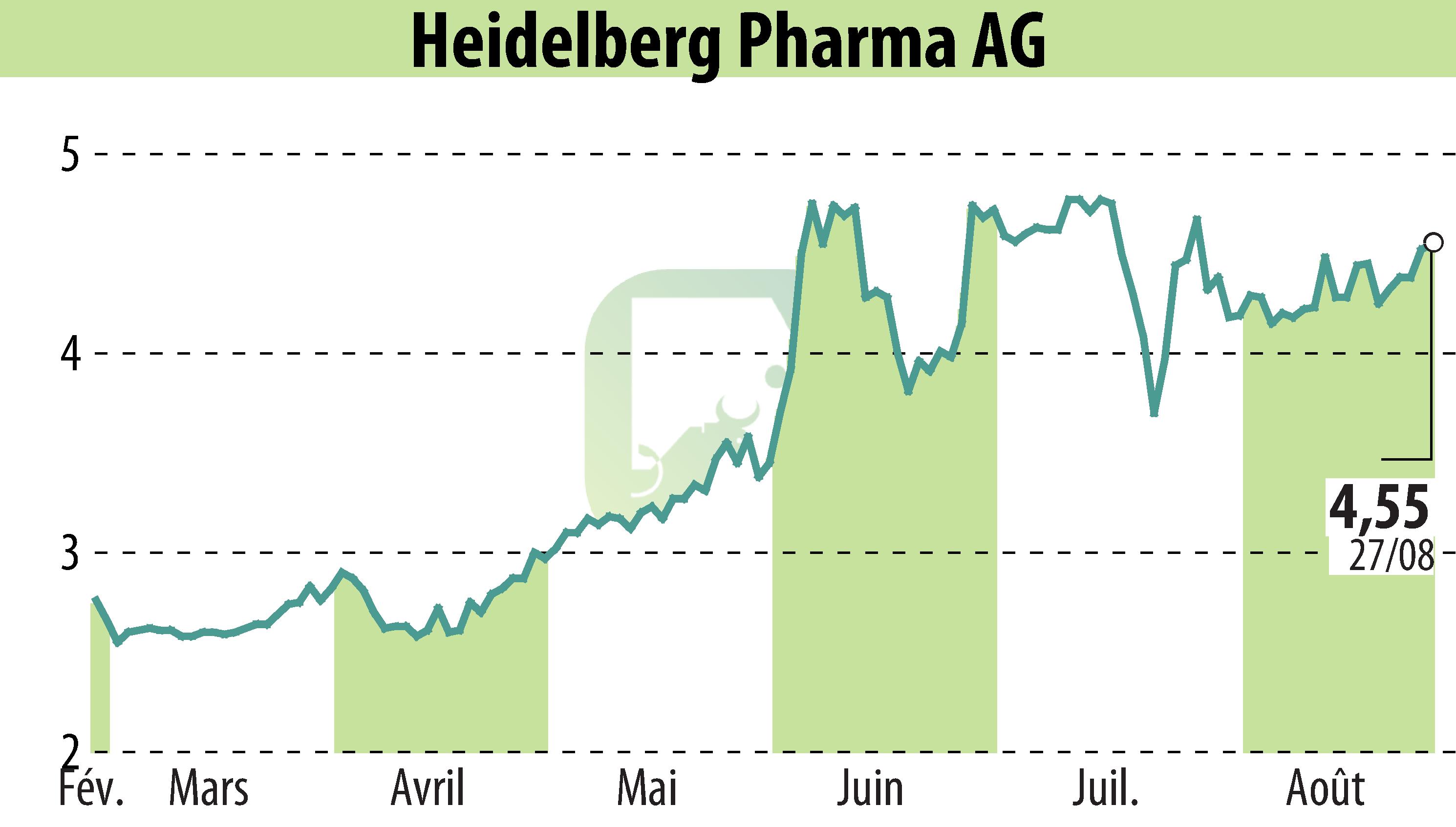
Heidelberg Pharma AG's partner, Telix Pharmaceuticals, encountered a regulatory setback concerning its imaging agent, TLX250-CDx. The FDA has raised concerns over the Chemistry, Manufacturing, and Controls (CMC) package, necessitating further data. This has resulted in a delay in a planned USD 70 million payment from HealthCare Royalty to Heidelberg Pharma.
Telix had submitted a Biologic License Application (BLA) for TLX250-CDx, which received a Priority Review. Despite initial acceptance, a Complete Response Letter was issued due to identified deficiencies. Telix is tasked with proving comparability between the drug used in the ZIRCON Phase 3 trial and the intended commercial product. Two third-party partners also need to address deficiencies.
This development affects Heidelberg Pharma's financial timeline, with cash reserves expected to last until the first quarter of 2026.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Heidelberg Pharma AG news
