on Heidelberg Pharma AG (ETR:HPHA)
Heidelberg Pharma's HDP-101 Receives Fast Track Designation from FDA
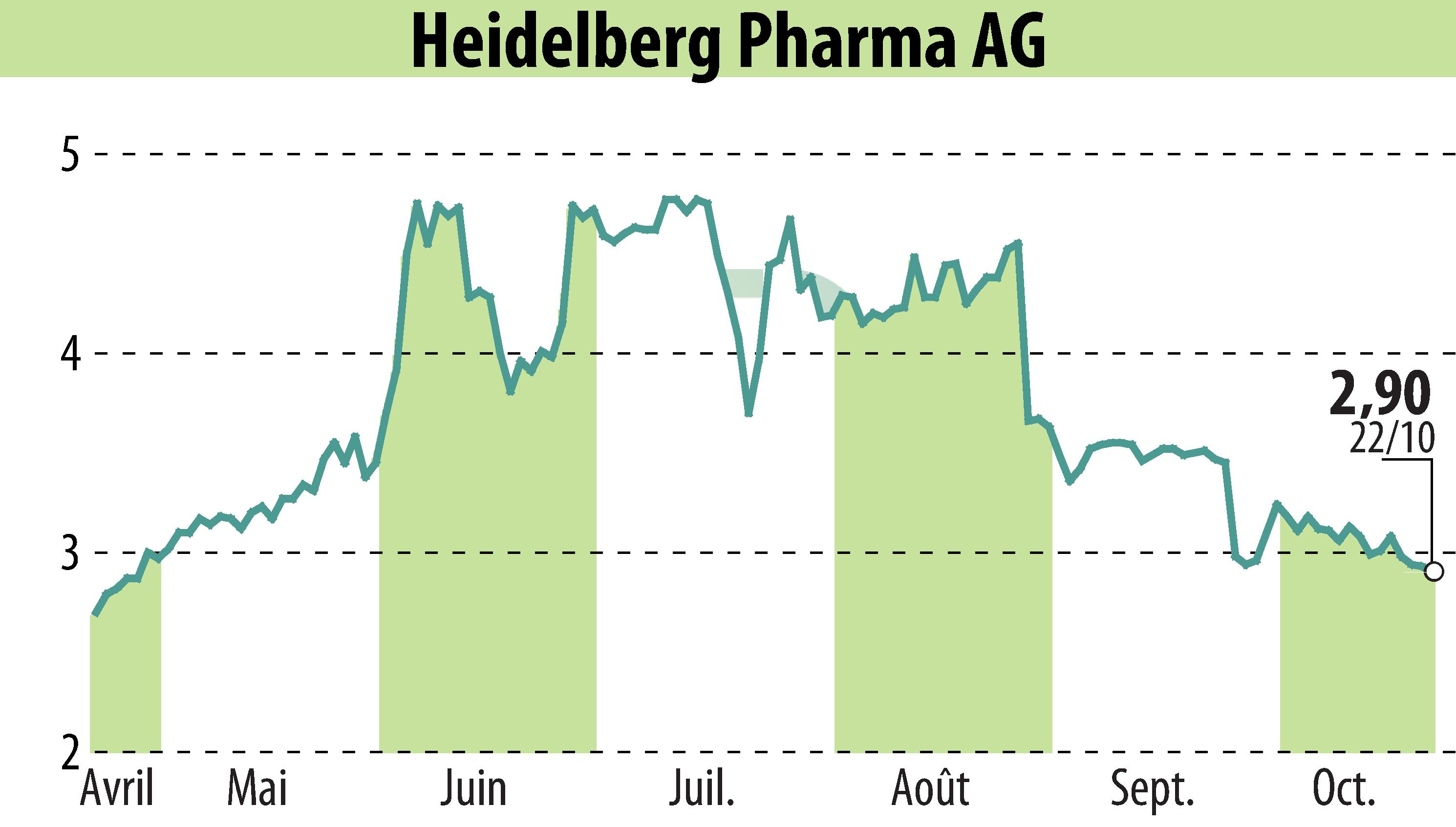
Heidelberg Pharma AG announced that its lead ADC candidate, HDP-101, has been granted Fast Track Designation by the US FDA for the treatment of multiple myeloma. This designation underscores the potential of HDP-101 to address significant unmet medical needs in treating serious conditions.
The Fast Track status enables more frequent interaction with the FDA and allows for a rolling review process, potentially expediting the development and review timeline. The designation is based on encouraging nonclinical and preliminary clinical data from an ongoing Phase I/IIa study evaluating HDP-101's safety and antitumor activity.
HDP-101 (INN: pamlectabart tismanitin) is still under investigation and has not yet received marketing approval from any regulatory authority. Heidelberg Pharma remains committed to advancing this promising candidate to provide new treatment options for patients with relapsed or refractory multiple myeloma.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Heidelberg Pharma AG news
