on Heidelberg Pharma AG (ETR:HPHA)
Heidelberg Pharma Begins Phase I Study for HDP-102 in Non-Hodgkin Lymphoma
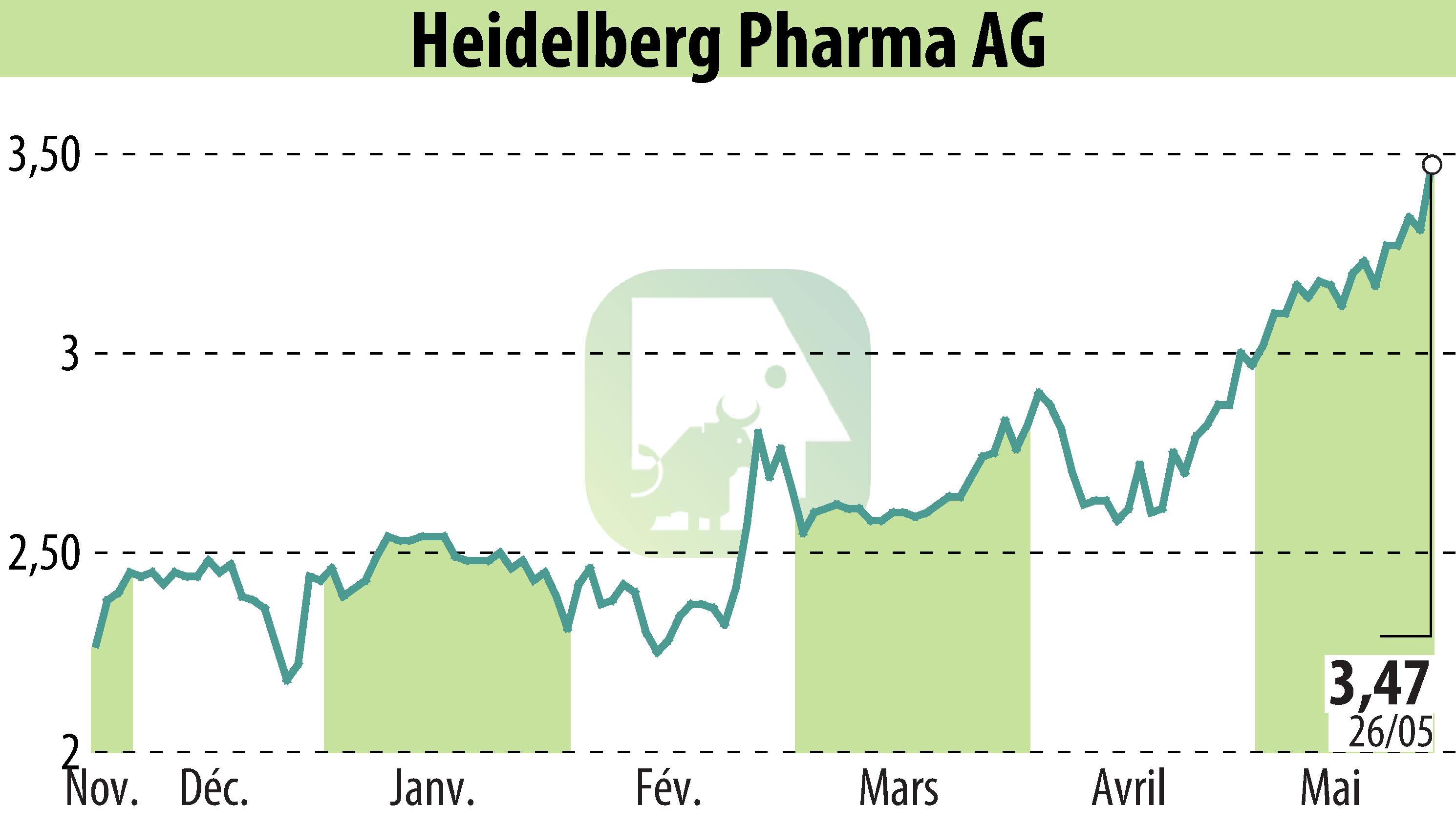
Heidelberg Pharma AG, a biotech firm from Ladenburg, Germany, announced the dosing of the first patient in a Phase I clinical trial for HDP-102. This treatment, designed for non-Hodgkin lymphoma (NHL), utilizes the company's Amanitin-based ADC technology. HDP-102 is the second drug from this platform to enter clinical development, following the promising HDP-101 trials on multiple myeloma.
The trial, now conducted in Moldova, assesses safety, tolerability, and more across multiple centers and countries including Israel and selected EU nations. Initial focus is on determining the optimal future dosage before expanding evaluation. HDP-102 targets the CD37 antigen found on certain B-cell lymphoma cells.
Heidelberg Pharma's preclinical data showed strong antitumor efficacy at notable conferences, supporting their ongoing advancement in addressing lymphoma treatment challenges.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Heidelberg Pharma AG news
