on Jaguar Health, Inc. (NASDAQ:JAGX)
FDA Extends Conditional Approval for Jaguar Health's Canalevia-CA1
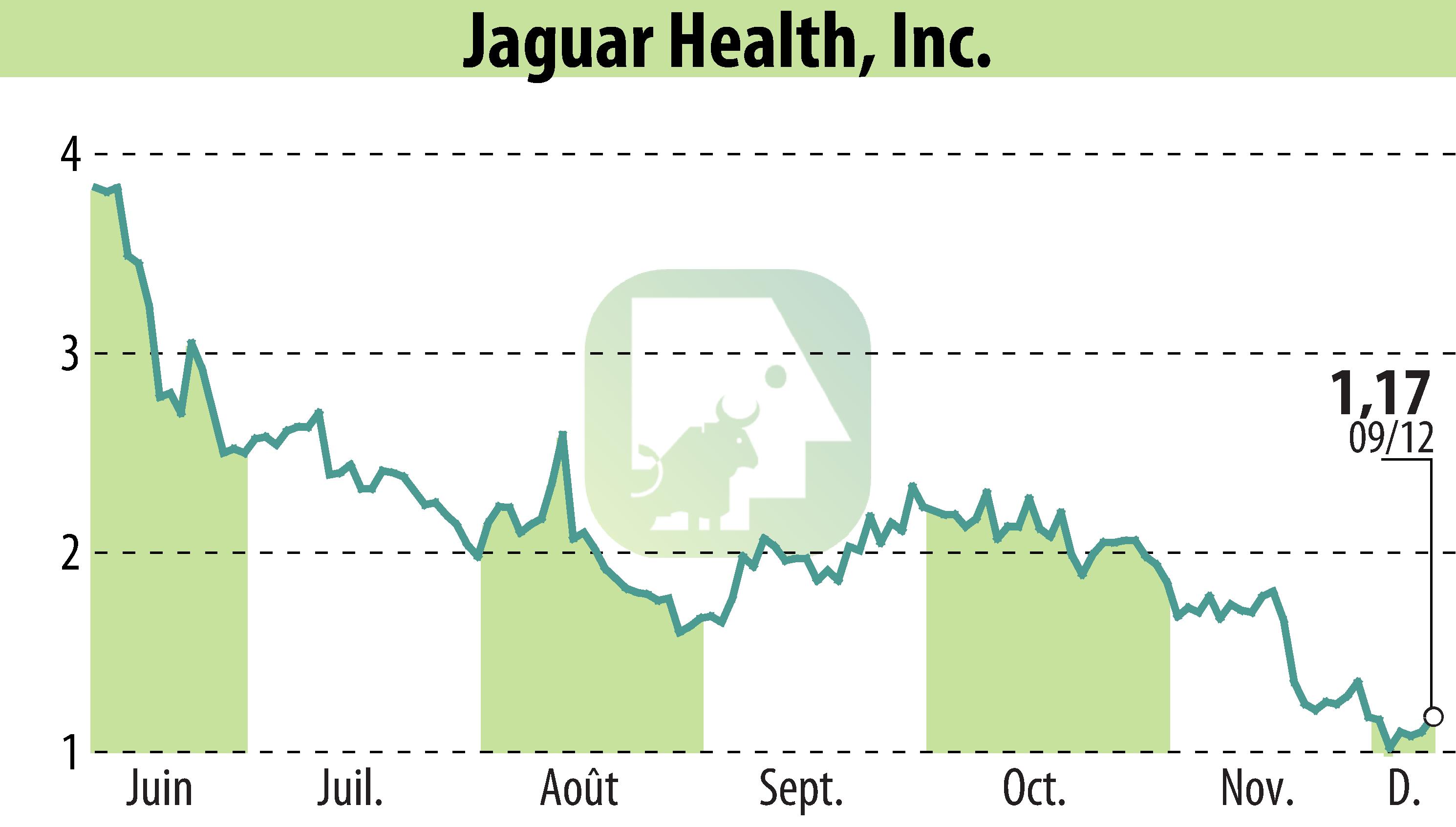
Jaguar Health, Inc. announced that the FDA has extended the conditional approval of Canalevia-CA1 for treating chemotherapy-induced diarrhea (CID) in dogs until December 2026. The drug, containing crofelemer, is available through major veterinary distributors such as Chewy.
The renewal represents the final allowable year of conditional approval, highlighting active progress toward the required evidence for full FDA approval. A confirmatory trial involving 100 dogs is underway, expected to conclude by February 2026, ahead of the FDA's June deadline.
With CID as a significant side effect in canine cancer treatments, Jaguar Health emphasizes the importance of Canalevia-CA1 for maintaining successful therapy outcomes. The move underscores the unmet need for effective treatments in veterinary medicine.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Jaguar Health, Inc. news
