on Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Initiates U.S. Trial for Crofelemer in SBS-IF Patients
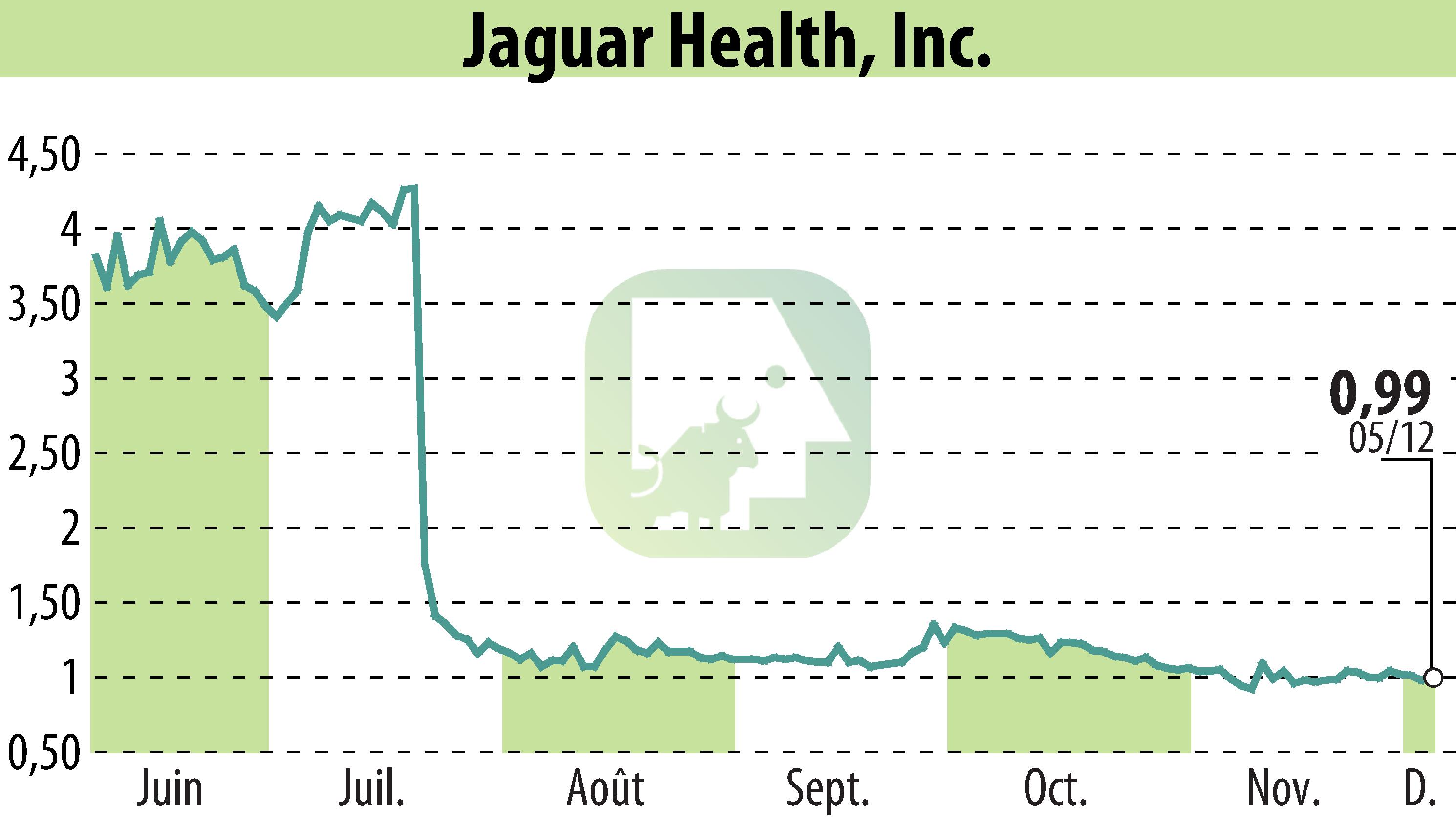
Jaguar Health, Inc. has launched a U.S.-based trial to evaluate the efficacy of crofelemer, a plant-based anti-diarrheal drug, for short bowel syndrome with intestinal failure (SBS-IF) in adults. This study is one of five clinical efforts across the U.S., EU, and MENA regions. Results from these proof-of-concept studies may be available in Q2 2025.
Crofelemer has Orphan Drug Designation by the FDA and European Medicines Agency for SBS-IF and microvillus inclusion disease (MVID), an ultrarare congenital diarrheal disorder. The studies aim to assess the quality of life impacts on patients and caregivers.
SBS-IF involves severe chronic diarrhea and necessitates intensive parenteral nutrition, which poses significant health challenges. Napo Pharmaceuticals, a Jaguar subsidiary, spearheads this initiative.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Jaguar Health, Inc. news
