on Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Presents Promising Trial Results for Pediatric Intestinal Failure Treatment
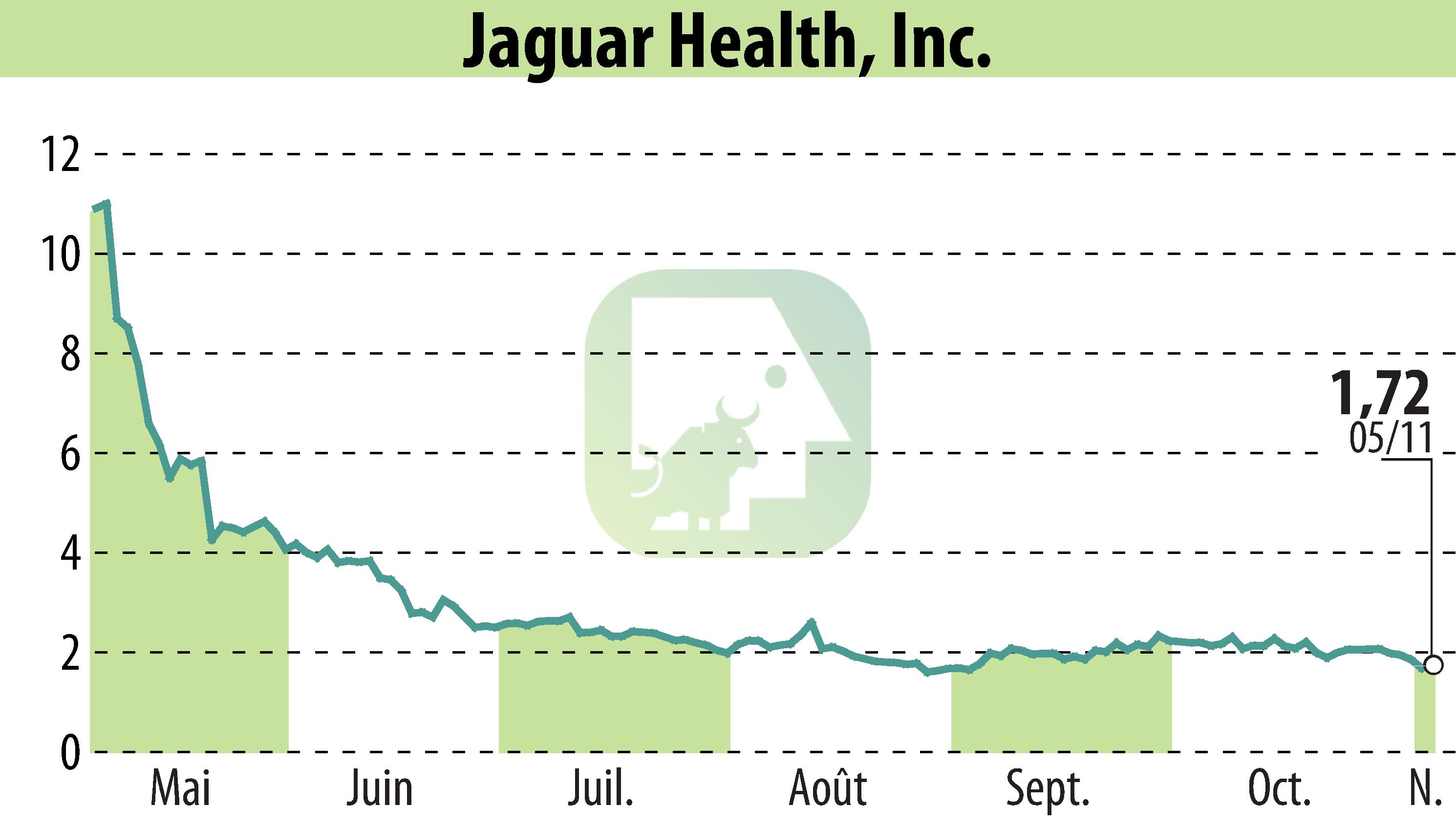
Jaguar Health, Inc. announced promising preliminary results from an ongoing trial of crofelemer for pediatric intestinal failure patients. Conducted in the UAE, the study demonstrated a reduction in parenteral support needs by 12% to 37%. This development marks a significant modification in disease progression for conditions such as microvillus inclusion disease (MVID) and short bowel syndrome (SBS-IF).
Currently, MVID affects 100-200 children globally, necessitating life-sustaining parenteral support due to severe malabsorption, while SBS affects approximately 10,000 to 20,000 people in the U.S. The unique mechanism of crofelemer offers a potential breakthrough in patient care and quality of life.
Jaguar is extending its trial efforts in the U.S., E.U., and Middle East and supports expanded access programs authorized by the FDA. The company continues to explore crofelemer's safety and efficacy in both pediatric and adult intestinal failure patients.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Jaguar Health, Inc. news
