on Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Secures $250,000 FDA Grant for Canalevia Trial
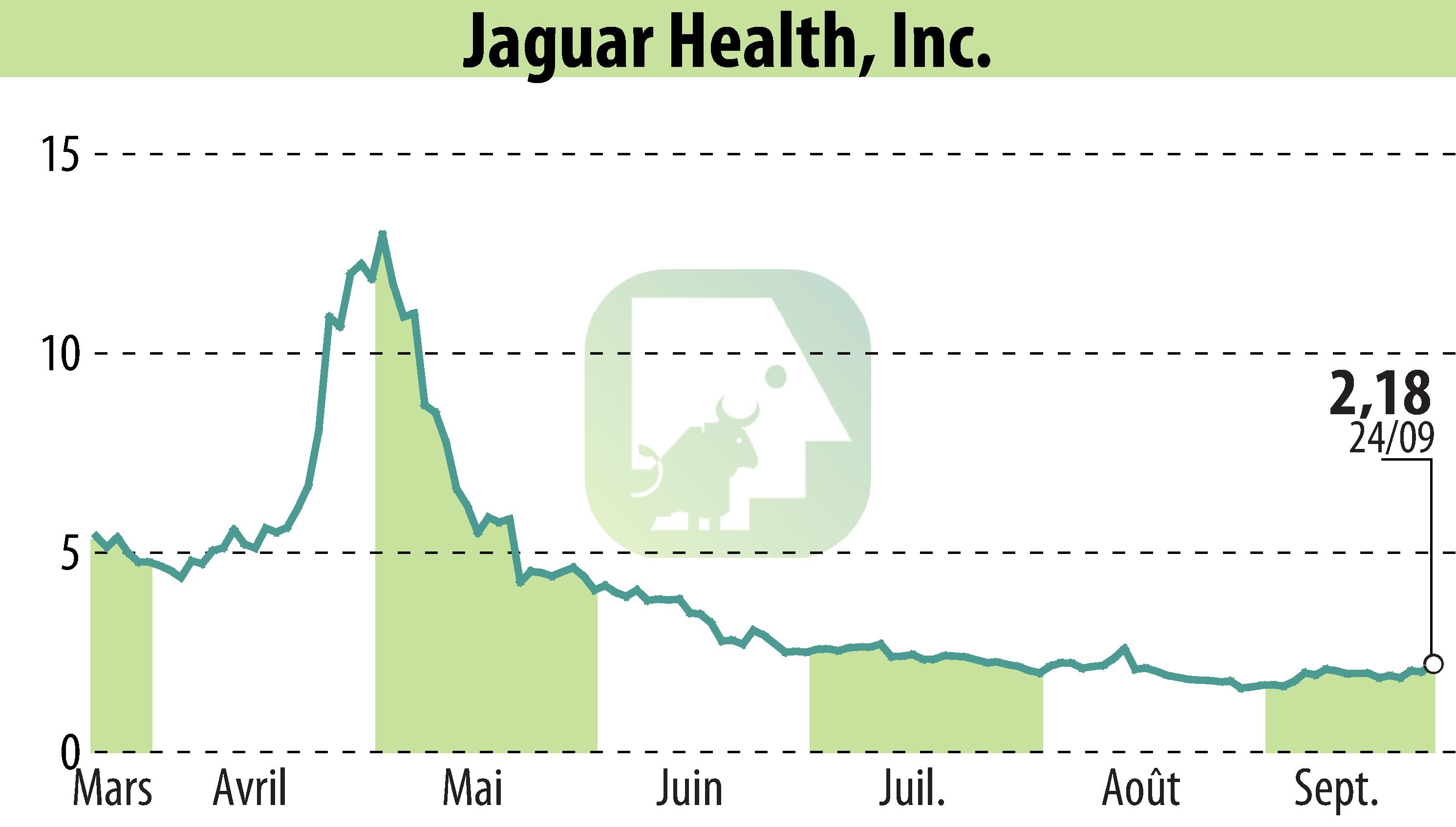
Jaguar Health, Inc. has announced receipt of a $250,000 grant from the FDA's Center for Veterinary Medicine. The grant is intended to support a confirmatory trial for Canalevia, aimed at securing full FDA approval for treating chemotherapy-induced diarrhea (CID) in dogs. Conditional approval for Canalevia was originally granted in December 2021. Jaguar is in discussions with animal health partners to expand Canalevia's use for general diarrhea treatment globally.
Lisa Conte, Jaguar's CEO, emphasized the company's strategy to partner for U.S. and EU approval of Canalevia for general diarrhea treatment in dogs. The drug, derived from plant sources, is not an antibiotic, reducing the risk of antibiotic resistance. Currently, no FDA-approved treatment exists for non-infectious diarrhea in dogs.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Jaguar Health, Inc. news
