on Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health's Crofelemer Shows Promise in Treating Cancer Therapy-Related Diarrhea
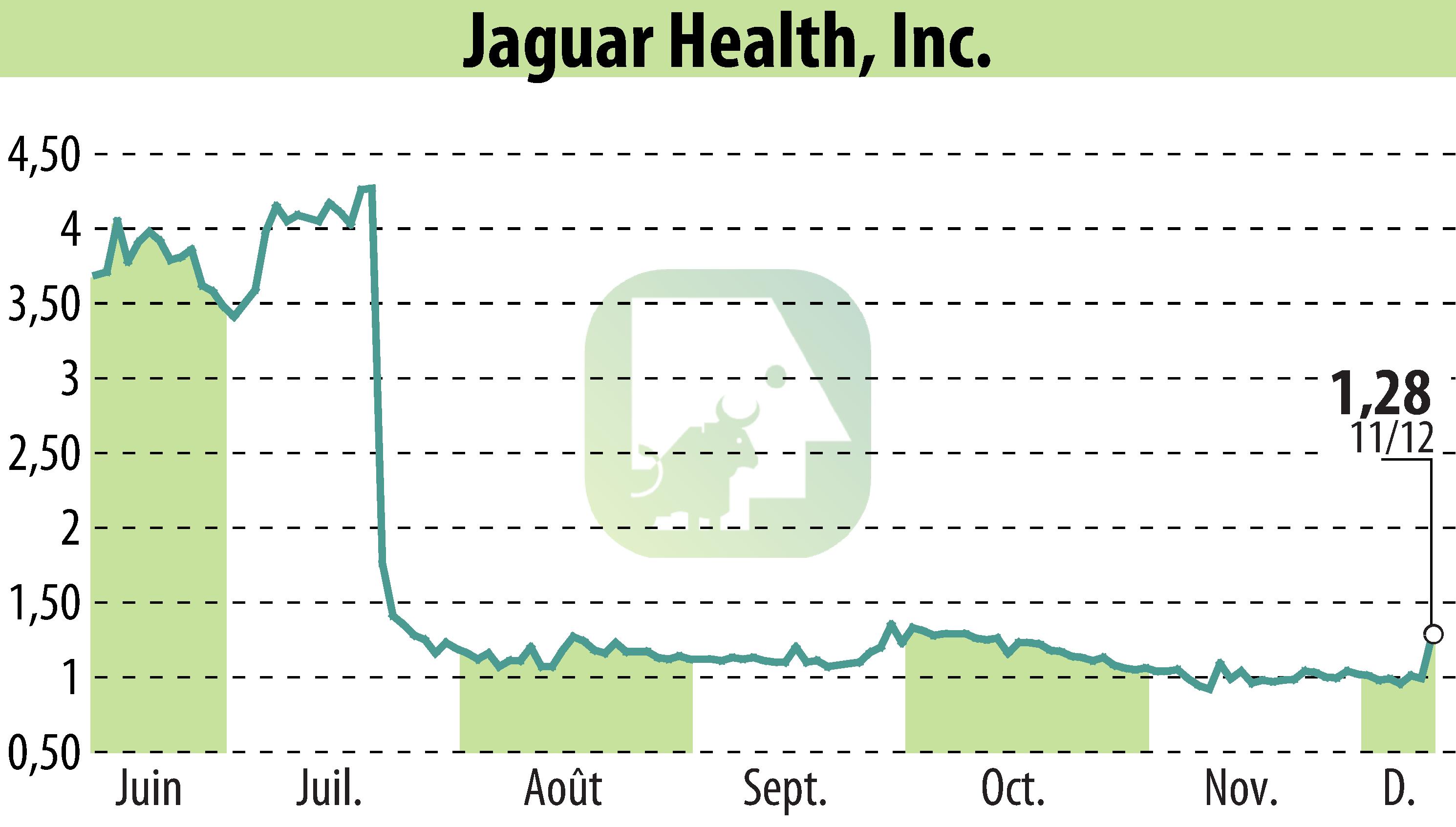
Jaguar Health has reported positive phase 3 trial outcomes for crofelemer in tackling cancer therapy-related diarrhea (CTD) among breast cancer patients. Findings were presented at the San Antonio Breast Cancer Symposium (SABCS). Crofelemer outperformed placebo in reducing diarrhea symptoms, enabling patients to maintain their cancer treatments. Significantly, 47.1% of crofelemer recipients were responders, compared to 33.7% in the placebo group. These results underscore crofelemer's potential in CTD management, crucial for treatment adherence and patient outcomes.
Diarrhea is common with therapies like abemaciclib and pertuzumab, often leading to dose adjustments. In the study, over 43% of abemaciclib users and all on pertuzumab required dose changes. Managing CTD could enhance patients' quality of life and treatment effectiveness. Jaguar Health plans to use these findings to engage with the FDA about crofelemer's potential pathway to availability for breast cancer patients. Further analyses of OnTarget trial data are expected to inform future submissions to scholarly forums.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Jaguar Health, Inc. news
