on MEDINCELL (EPA:MEDCL)
FDA Approves UZEDY for Bipolar I Disorder in Adults
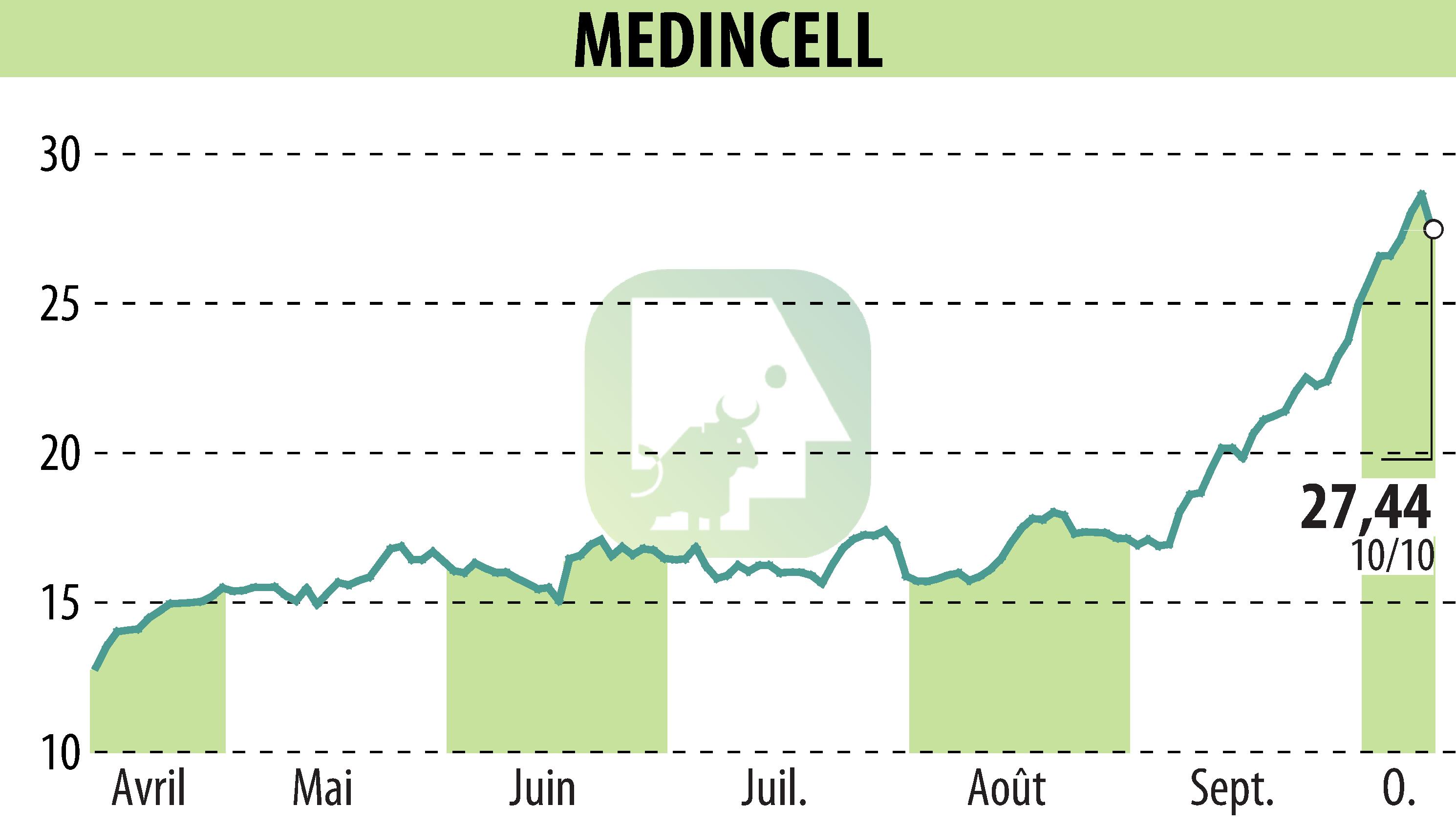
The FDA has approved UZEDY® (risperidone) extended-release injectable suspension for use as monotherapy or adjunctive therapy for adults with bipolar I disorder. This formulation is already approved for treating schizophrenia in adults, marking a significant development in psychiatric care. UZEDY, the first long-acting, subcutaneous risperidone, employs Medincell's proprietary SteadyTeq™ technology, ensuring controlled release of the medication.
Chris Fox from Teva emphasized the importance of this approval in addressing unmet treatment needs for those living with bipolar I disorder. Notably, around 1% of U.S. adults are estimated to develop this disorder. The approval relies on past clinical data and innovative development methodologies, enhancing treatment adherence, a critical need in mental health care.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all MEDINCELL news
