on Moderna, Inc. (NASDAQ:MRNA)
EMA Recommends Moderna's Updated COVID-19 Vaccine for EU Authorization
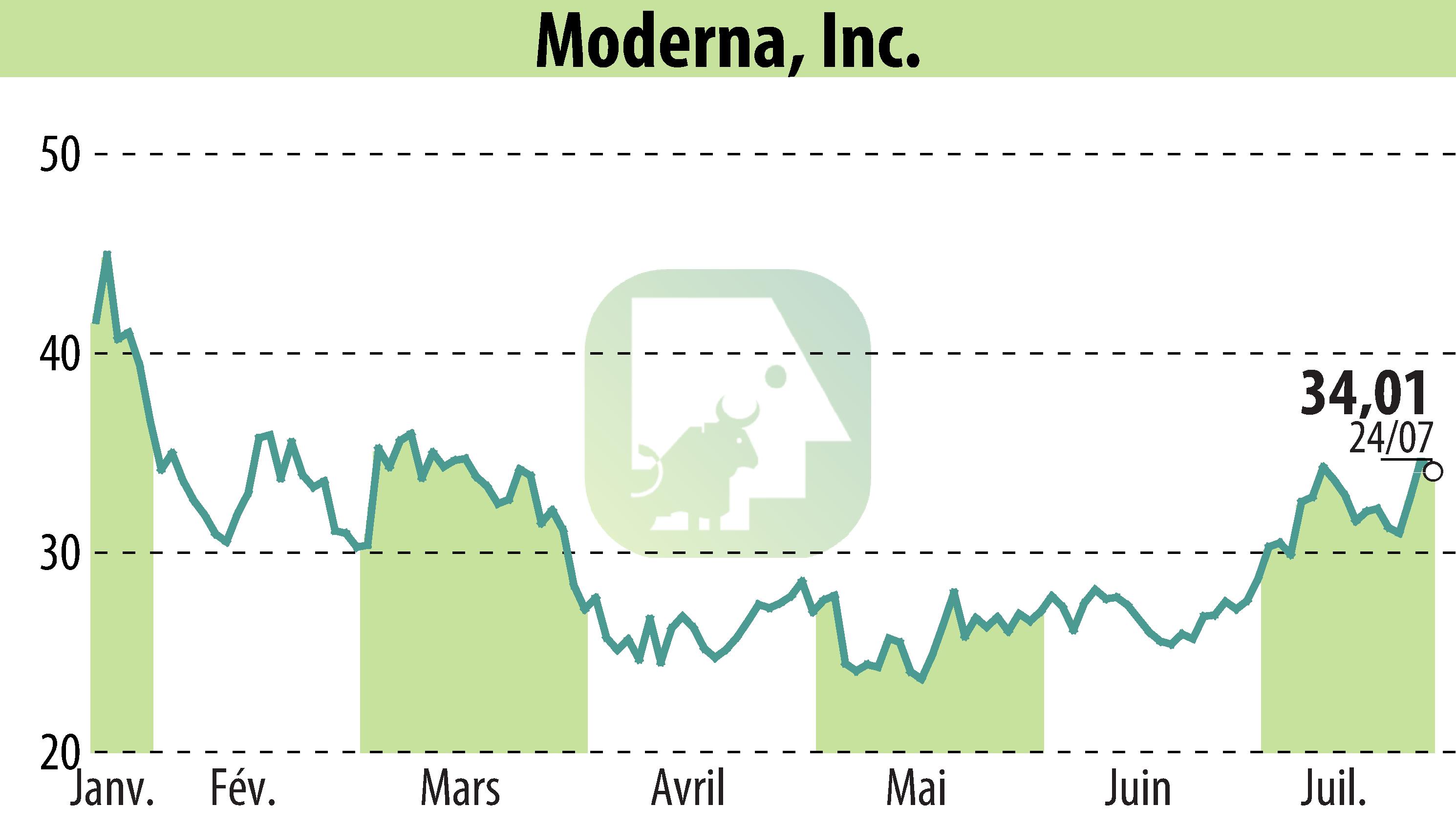
Moderna, Inc. has announced a positive recommendation from the European Medicines Agency's Committee for Medicinal Products for Human Use regarding their updated COVID-19 vaccine. This formulation targets the SARS-CoV-2 variant LP.8.1 and is intended for individuals aged six months and older. Pending authorization from the European Commission, the vaccine could be available for the 2025-2026 vaccination season.
CEO Stéphane Bancel highlighted this development as a significant step in addressing the ongoing threat of COVID-19, particularly among vulnerable populations. The decision by the CHMP is based on various data and aligns with global health guidelines identifying LP.8.1 as a pertinent vaccine update.
Moderna is also pursuing regulatory approvals for this vaccine variant worldwide.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
