on Moderna, Inc. (NASDAQ:MRNA)
Moderna Receives Health Canada Approval for Updated COVID-19 Vaccine
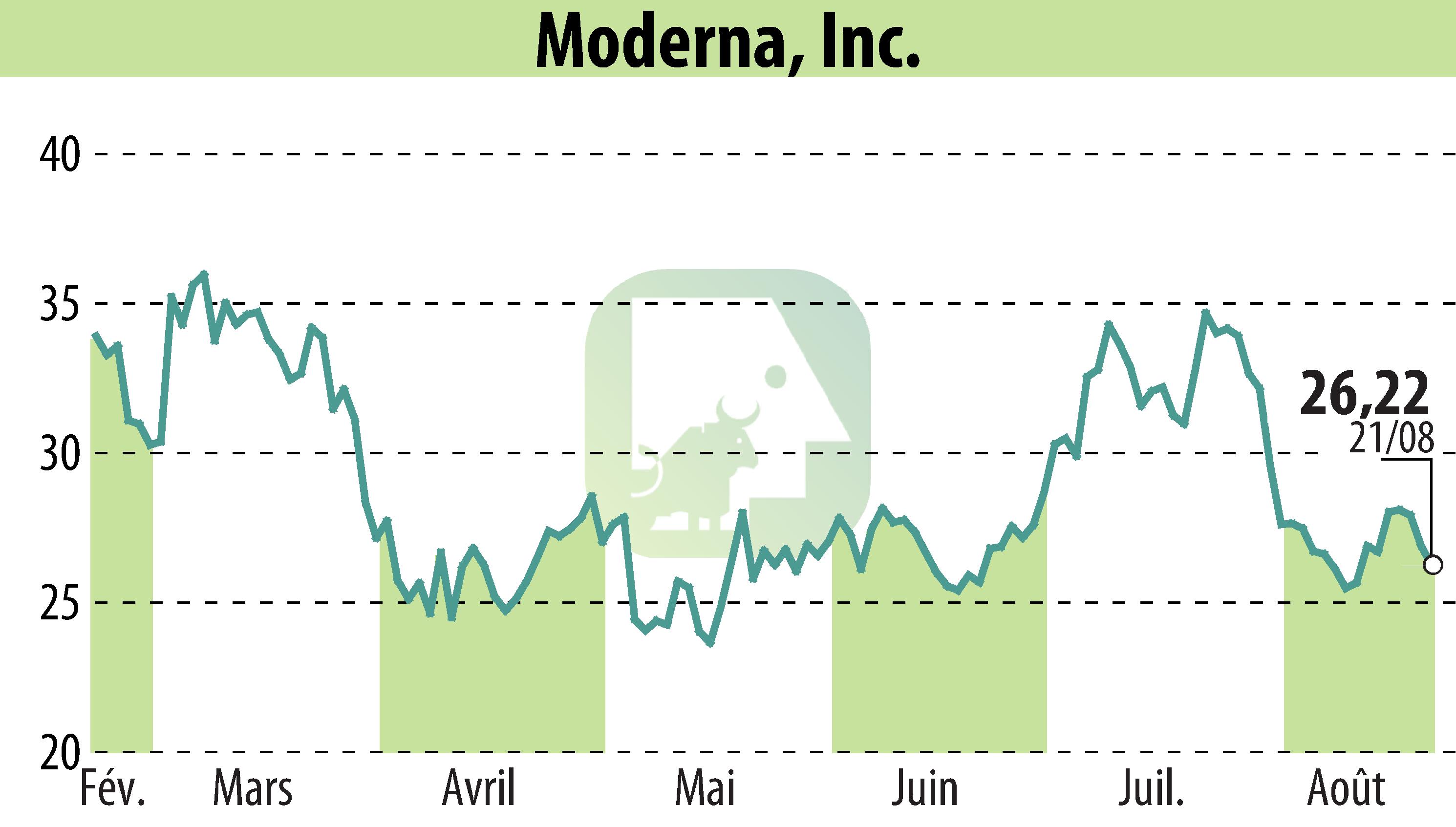
Moderna has announced Health Canada's approval of its updated Spikevax® COVID-19 mRNA vaccine, which targets the SARS-CoV-2 LP.8.1 variant. This vaccine is available for individuals aged six months and older and will be delivered in time for the 2025-2026 vaccination season. Notably, all Spikevax pre-filled syringe doses for Canada will now be produced domestically, a first for the Canadian market.
Production will take place at Moderna's new facility in Laval, Quebec, with final operations by Novocol Pharma in Cambridge, Ontario. These Canadian-made doses are expected to be available this fall. Stéphane Bancel, CEO of Moderna, describes the approval as a testament to Canada's growing biomanufacturing leadership.
Already approved in Europe, Japan, and Switzerland, the updated vaccine is under regulatory review in several other countries. Each Canadian province and territory will set eligibility for the public vaccination program. For those not covered, efforts are being made to streamline access through private insurers.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
