on Moderna, Inc. (NASDAQ:MRNA)
Moderna's Updated COVID-19 Vaccines Approved by U.S. FDA
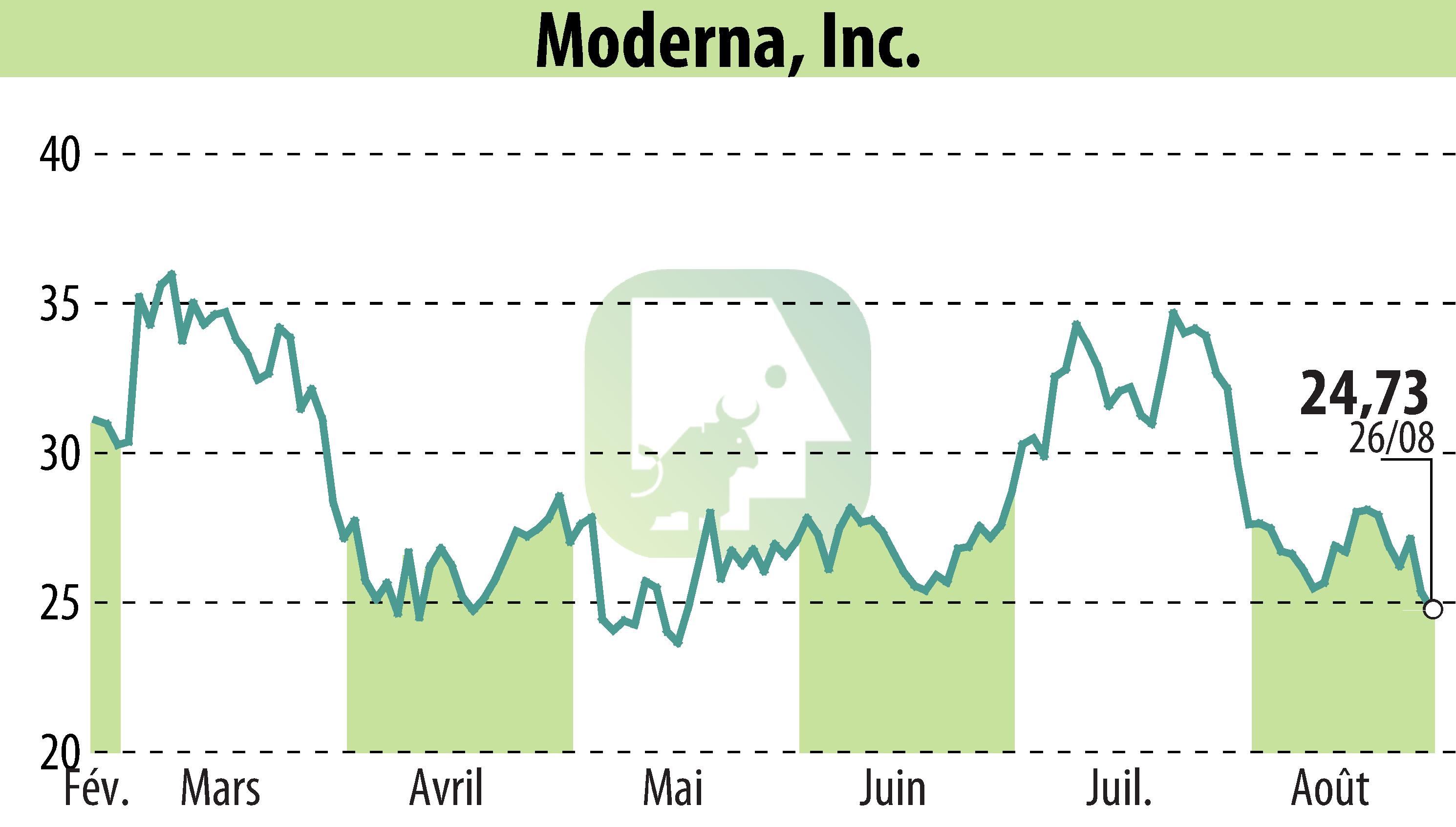
Moderna, Inc. has announced that the U.S. FDA has approved its supplemental Biologics License Applications for their 2025-2026 Spikevax® and mNEXSPIKE® vaccines. These updated vaccines target the LP.8.1 variant of SARS-CoV-2 and are intended to prevent COVID-19, especially in high-risk groups.
The updated Spikevax formula is approved for those 6 months to 64 years with underlying conditions, and all individuals aged 65 and older. Similarly, mNEXSPIKE is approved for those 12 to 64 years with underlying health risks, and everyone 65 and above. These vaccines should be available shortly following FDA clearance.
The updated vaccine formula follows U.S. FDA guidance focusing on the LP.8.1 variant. The approval extends to other regions, including Canada and Europe, with more applications under review globally.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
