on Moderna, Inc. (NASDAQ:MRNA)
Moderna Reports Positive Phase 3 Results for Seasonal Influenza Vaccine
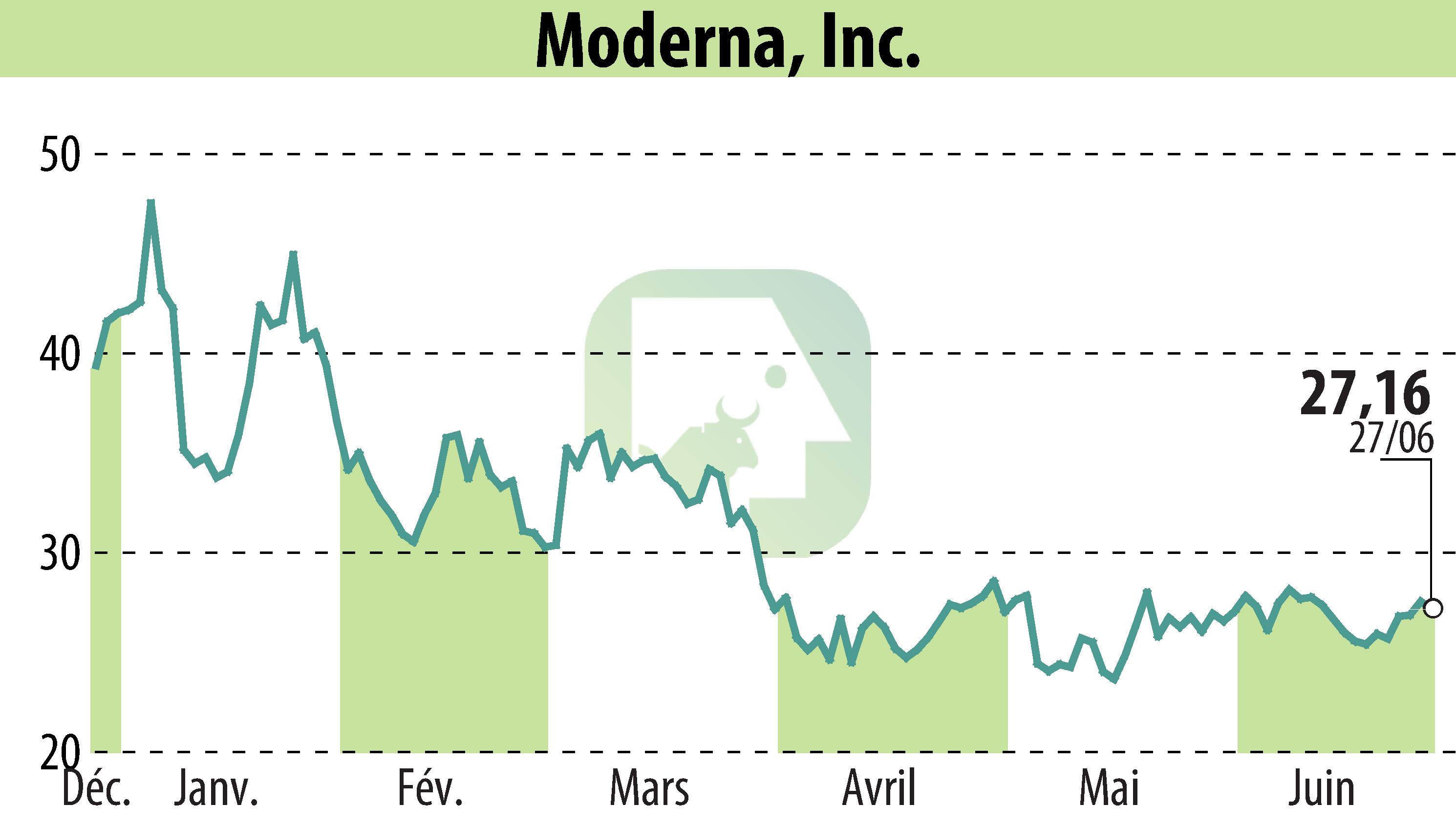
Moderna, Inc. has announced positive Phase 3 results for its seasonal influenza vaccine candidate, mRNA-1010. The study, focusing on adults aged 50 and older, demonstrated that mRNA-1010 had a relative vaccine efficacy (rVE) 26.6% higher than a licensed standard-dose vaccine. Efficacy was observed against all influenza strains included in the vaccine such as A/H1N1, A/H3N2, and B/Victoria.
The trial involved over 40,000 participants from 11 countries, showing mRNA-1010's promise in reducing influenza burden in older adults. Safety and tolerability were consistent with previous studies, with mild adverse reactions reported. Moderna plans to engage with regulators to advance filing submissions for the vaccine.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
