on Nanohale AG (ETR:FYB)
Formycon Launches Europe’s First Ranibizumab Biosimilar in Pre-filled Syringe
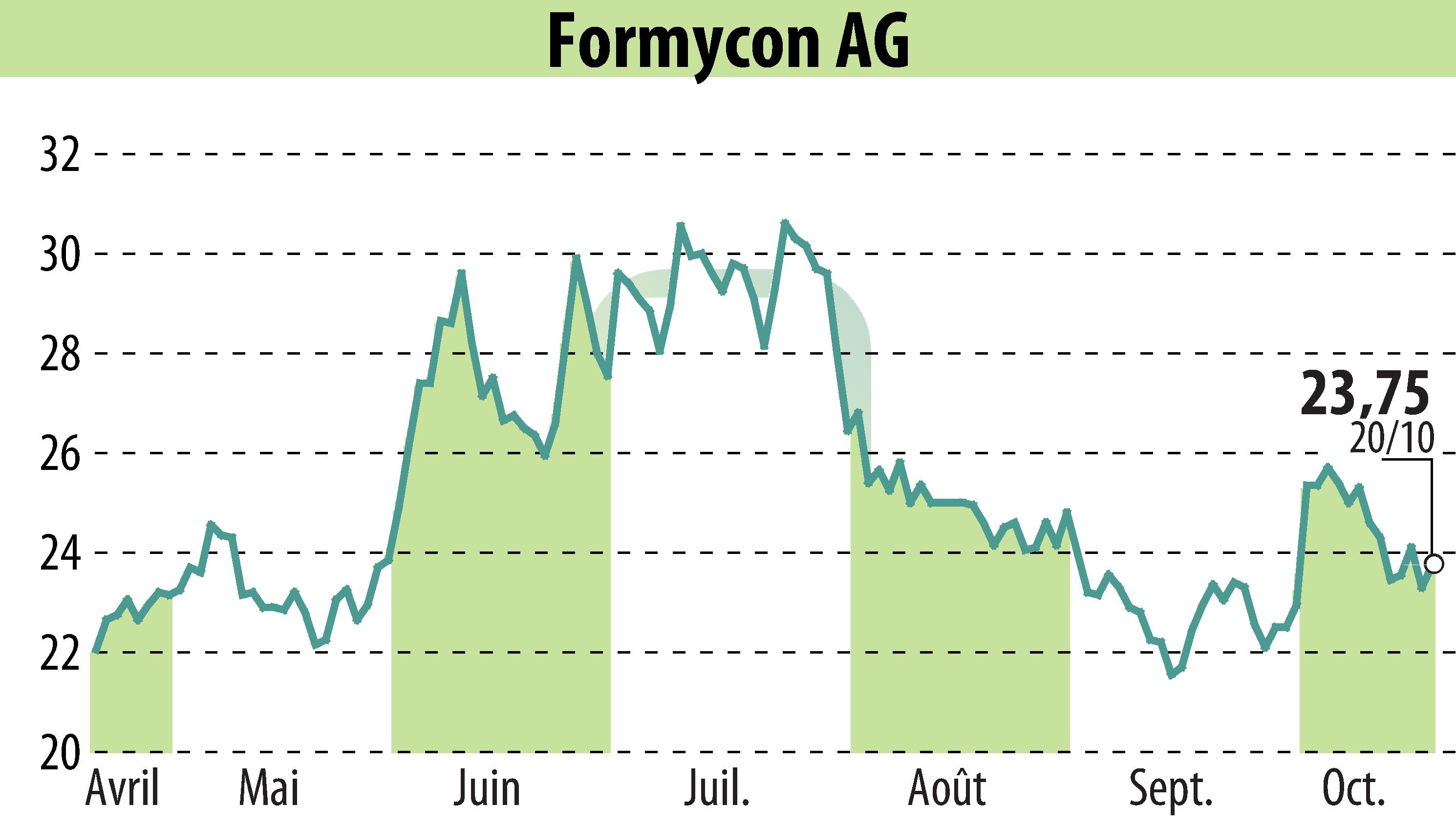
Formycon AG has introduced FYB201/Ranivisio®, the first ranibizumab biosimilar available in Europe in a pre-filled syringe. This innovative launch promises improved handling and dosing accuracy. Teva Pharmaceutical will commercialize the product across Europe, starting in France from October 2025, with Germany and other countries to follow.
FYB201/Ranivisio® enhances therapeutic options for treatments like wet age-related macular degeneration (nAMD). The syringe's design minimizes application errors, offering convenience to healthcare providers. This launch marks a significant step in ophthalmic care efficiency, expanding Formycon's reach in the biosimilar market.
The partnership with Teva leverages its extensive distribution network, fostering broader access to this novel treatment across Europe. This collaboration underscores Teva’s commitment to expanding its biosimilar portfolio, delivering effective therapies to patients.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
