on Nanohale AG (ETR:FYB)
Teva Partners with Formycon for FYB203 Biosimilar in Europe and Israel
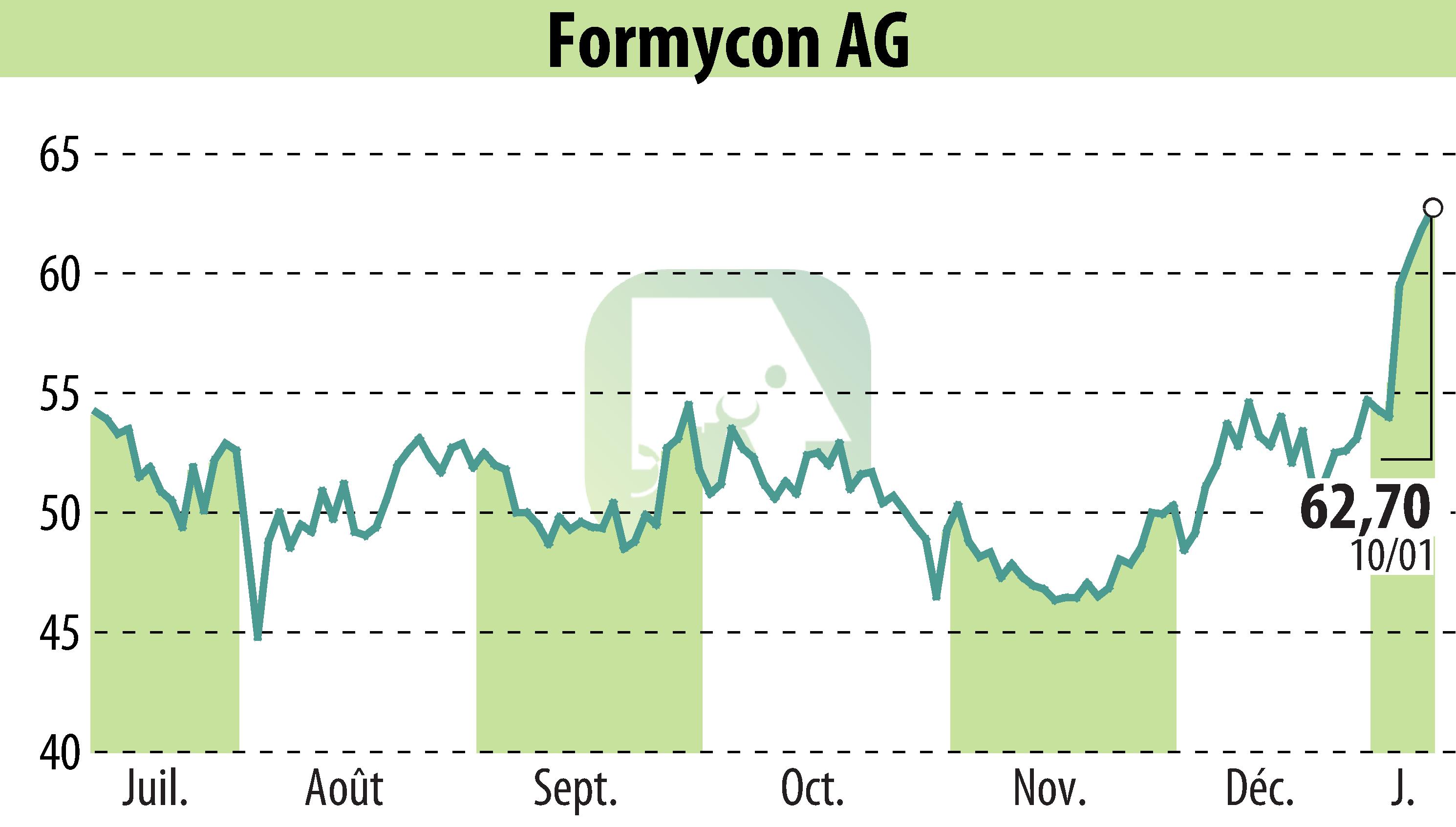
Teva Pharmaceuticals has become the strategic commercialization partner for Formycon's biosimilar candidate FYB203, targeting Eylea® (Aflibercept), in major parts of Europe and Israel. This follows a licensing agreement between Klinge Biopharma GmbH, the global rights holder for FYB203, and Teva Pharmaceuticals International GmbH, a Teva subsidiary. Formycon will provide the finished product to Teva under a separate agreement.
The partnership leverages Formycon's expertise in developing biosimilars and Teva's extensive commercial and distribution network across Europe. Teva will market FYB203 under the brand name AHZANTIVE®, pending regulatory approval, while Klinge is set to receive milestone payments and royalties on sales.
This collaboration aims to expand access to innovative medicines. FYB203 has received a positive recommendation from the European Medicines Agency, with a decision expected soon. Eylea®, used for treating severe retinal diseases, had approximately USD 9 billion in global sales in 2023.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
