on ORPHAN SYNERGY EUROPE-PHARMA (EPA:OSE)
FDA Grants Orphan Drug Status to OSE Immunotherapeutics' Pegrizeprument
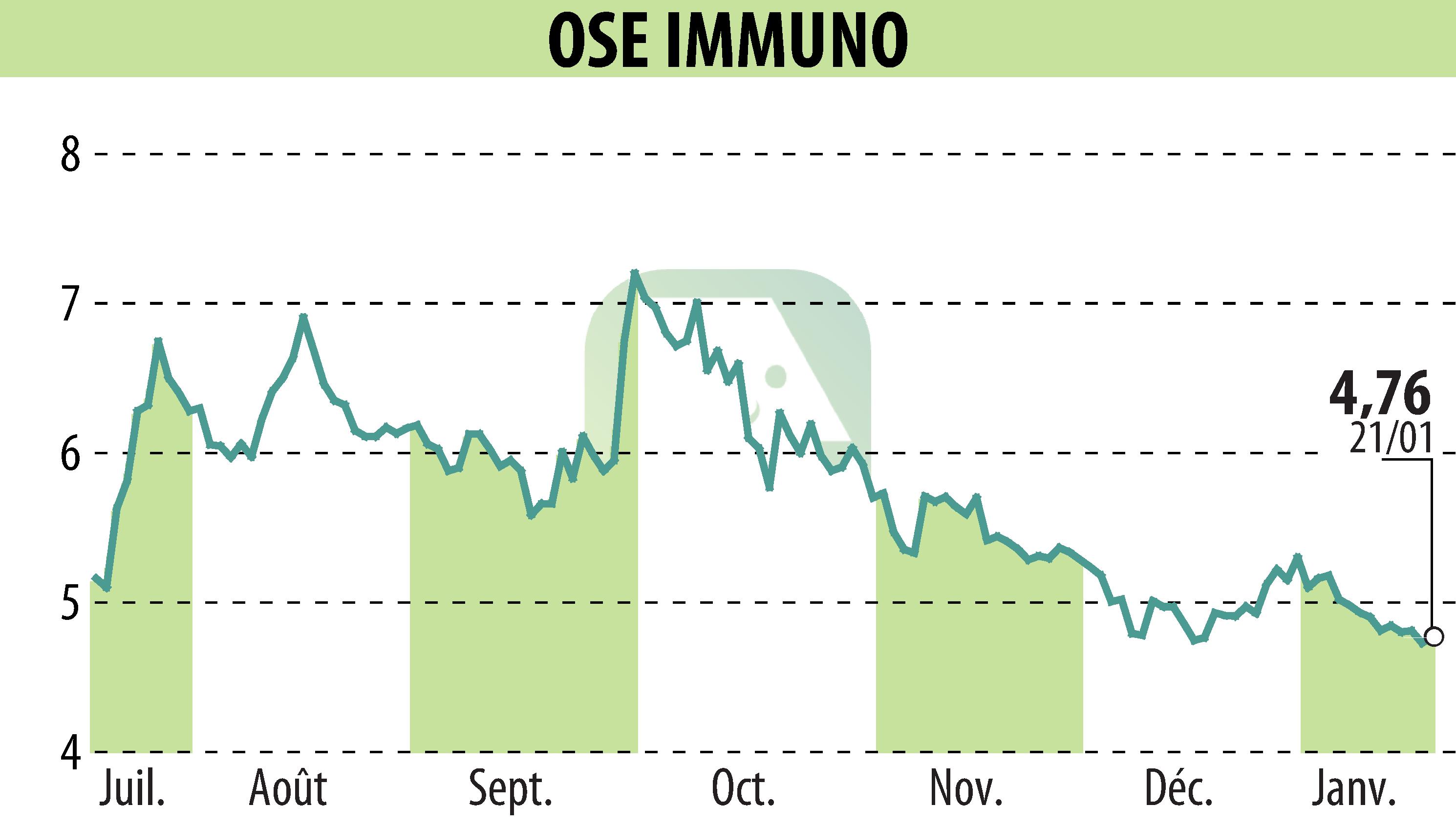
OSE Immunotherapeutics has announced that its partner, Veloxis Pharmaceuticals, has achieved a key regulatory milestone. The U.S. Food and Drug Administration (FDA) has conferred Orphan Drug Designation upon pegrizeprument (VEL-101), intended for preventing organ rejection in liver transplant patients. This antibody fragment, initially developed by OSE, was licensed to Veloxis in 2021. Veloxis will oversee its development and commercialization.
The orphan status highlights a severe need in solid organ transplantation, a point emphasized by OSE's Chief Development Officer, Sonya Montgomery. The designation aims to foster innovative treatments for rare conditions, affecting under 200,000 people in the U.S., by offering incentives to drug developers.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all ORPHAN SYNERGY EUROPE-PHARMA news
