on POXEL (EPA:POXEL)
TWYMEEG® Data Unveiled at Japan Diabetes Society Meeting
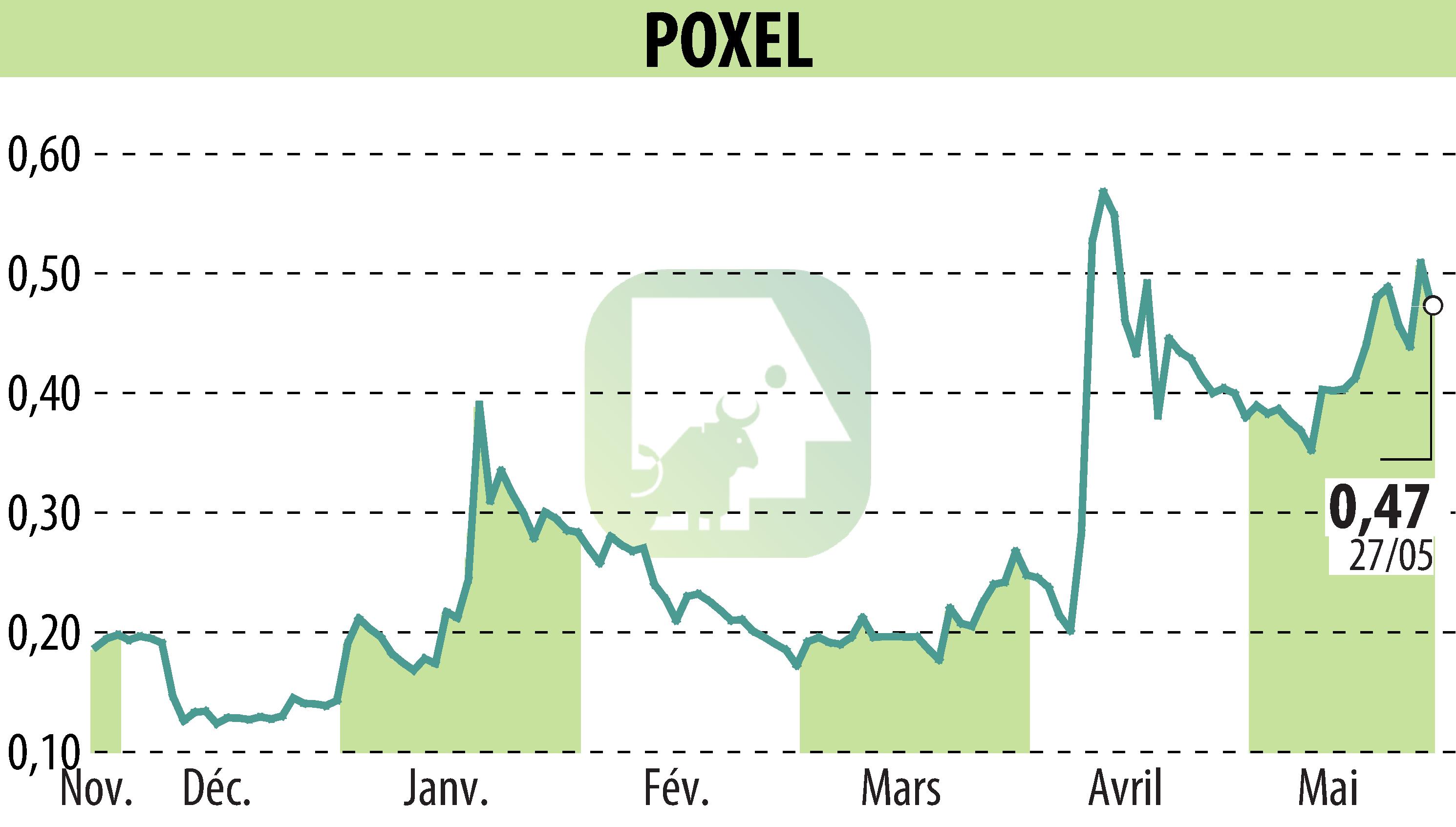
POXEL SA announced that new clinical and scientific data on TWYMEEG® will be presented at the 68th Annual Meeting of the Japan Diabetes Society, taking place May 29-31, 2025. The presentations include 15 studies supported by Sumitomo Pharma, involving 7 clinical trials, 3 post-hoc analyses, and 5 non-clinical studies.
The data confirm TWYMEEG®’s efficacy and safety in monotherapy and combination therapies. Key studies include the TWINKLE study, emphasizing its safety in diabetic patients with renal impairment, and the FAMILIAR study, highlighting efficacy when combined with DPP-4 inhibitors. Other findings focus on TWYMEEG®’s dual mechanism of insulin sensitivity and glucose excretion.
Thomas Kuhn, CEO of Poxel, expressed pride in the ongoing scientific interest in TWYMEEG® and its contribution to the product's clinical and commercial advancements in Japan.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all POXEL news
