on Protagonist Therapeutics, Inc. (NASDAQ:PTGX)
Protagonist Therapeutics Reports Promising Data for Icotrokinra in Ulcerative Colitis
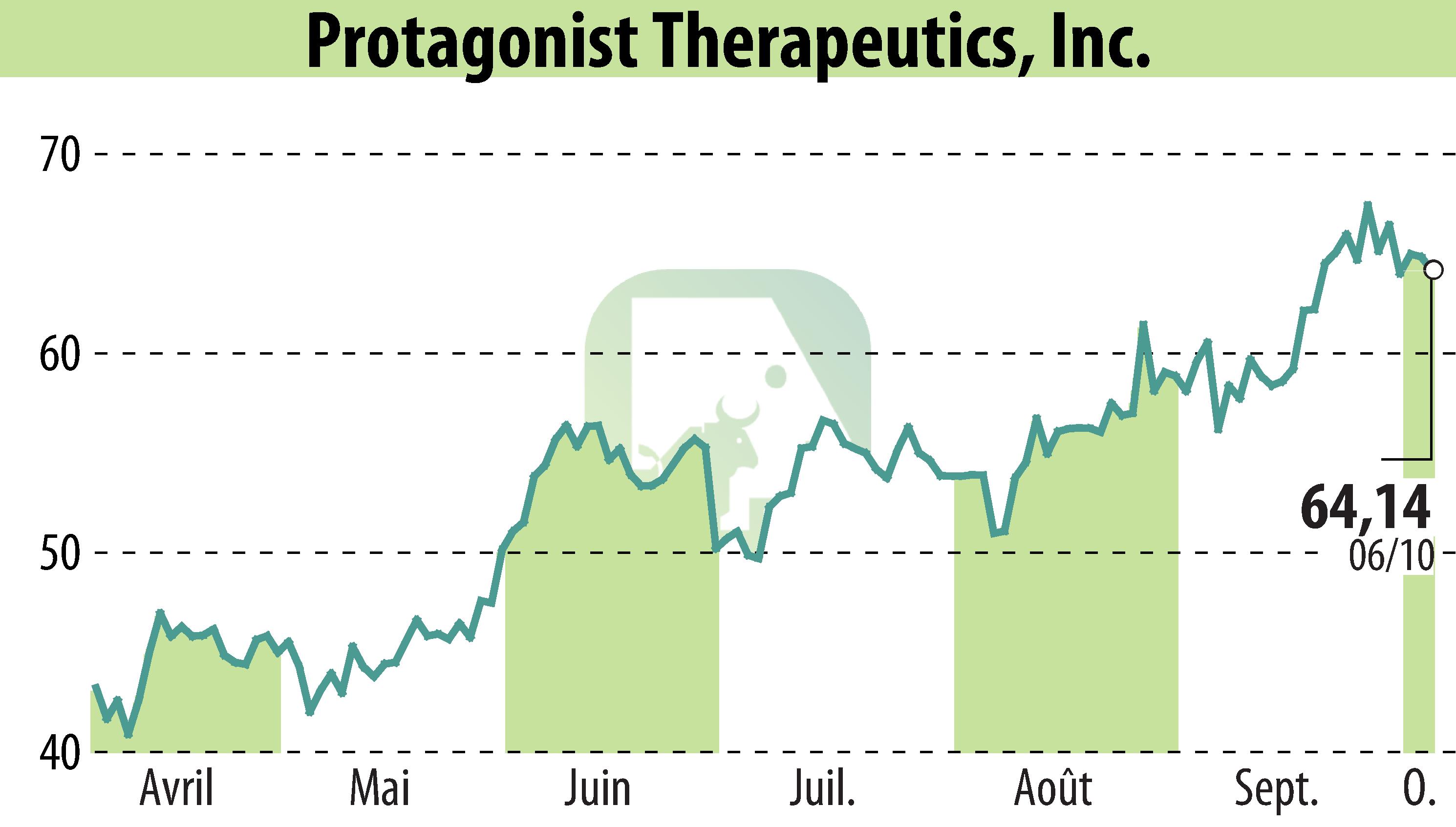
Protagonist Therapeutics has announced positive results from its Phase 2b ANTHEM-UC study of icotrokinra in adults with moderate to severe ulcerative colitis. The study achieved its primary endpoint of clinical response at all doses, with a significant 36.5% of patients showing endoscopic improvement. At the highest dose, 30.2% reached clinical remission by Week 12.
The findings highlight icotrokinra's potential as a first-in-class oral peptide targeting the IL-23 receptor, offering new hope for patients. The promising data paves the way for a Phase 3 trial in ulcerative colitis expected to commence in late 2025.
Patients on the 400 mg dose showed a 63.5% clinical response rate compared to 27% on placebo. The safety profile was comparable across all groups, suggesting a favorable balance of efficacy and safety.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Protagonist Therapeutics, Inc. news
