on Protagonist Therapeutics, Inc. (NASDAQ:PTGX)
Protagonist Therapeutics' Phase 3 Trial Data Shows Positive Results for Psoriasis Treatments
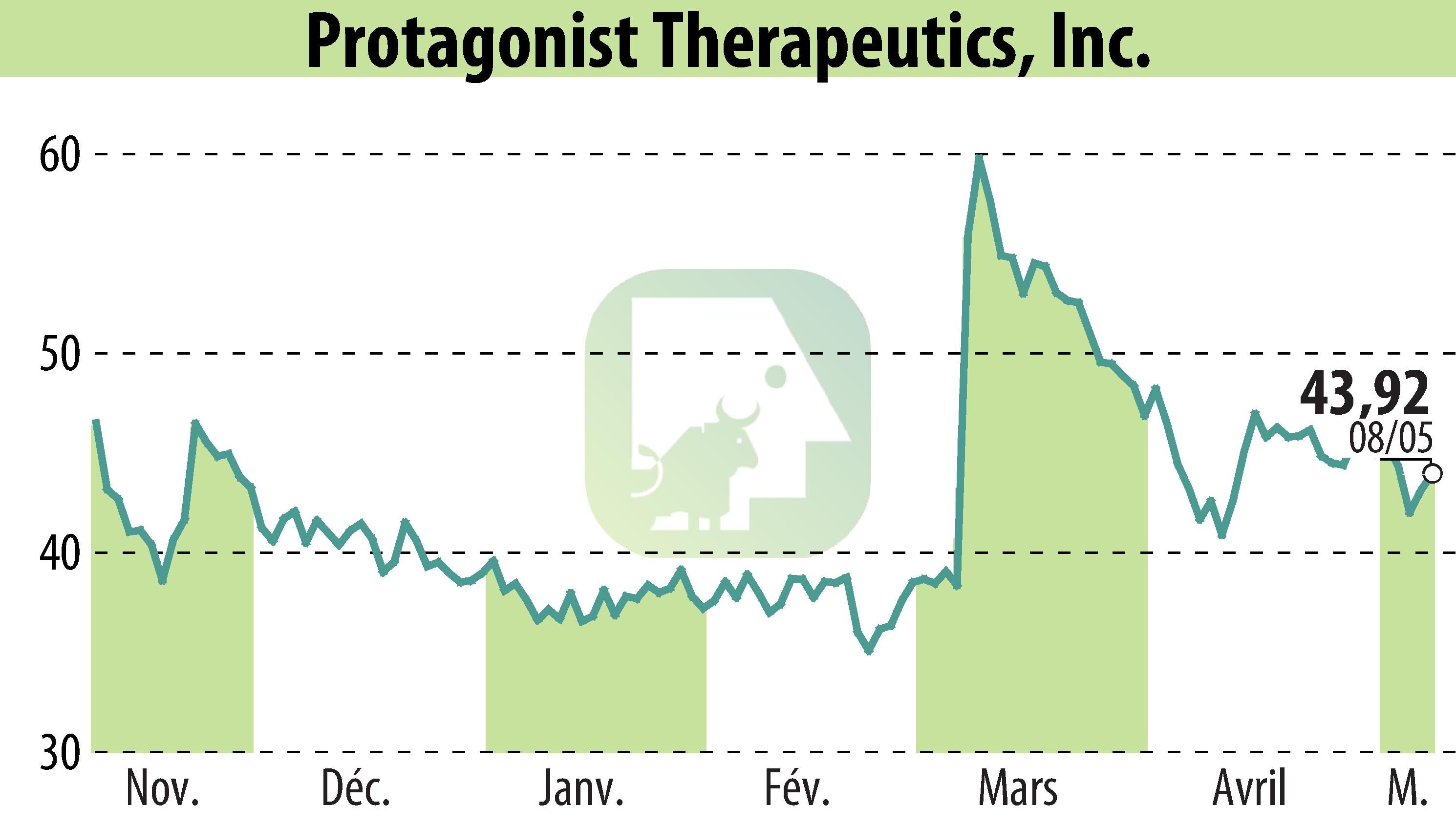
Protagonist Therapeutics has revealed promising results from its Phase 3 study, ICONIC-TOTAL, focused on icotrokinra for treating difficult psoriasis sites. The data showed significant improvements in patients with scalp and genital psoriasis. At 16 weeks, 66% achieved a clear or almost clear scalp and 77% showed clear genitals, compared to lower placebo results. Icotrokinra, a once-daily oral peptide, demonstrated both effectiveness and safety.
Additionally, Protagonist shared preclinical findings on PN-881, another innovative peptide targeting the IL-17 pathway. PN-881 has shown strong potency and stability, marking it as a robust candidate for oral delivery. This research was highlighted at the Society for Investigative Dermatology Annual Meeting. The study advances Protagonist's efforts to enhance psoriasis treatments and explores further clinical studies for these promising therapies later in 2025.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Protagonist Therapeutics, Inc. news
