on SANOFI-AVENTIS (EPA:SAN)
FDA Approves Dupixent for Bullous Pemphigoid Treatment
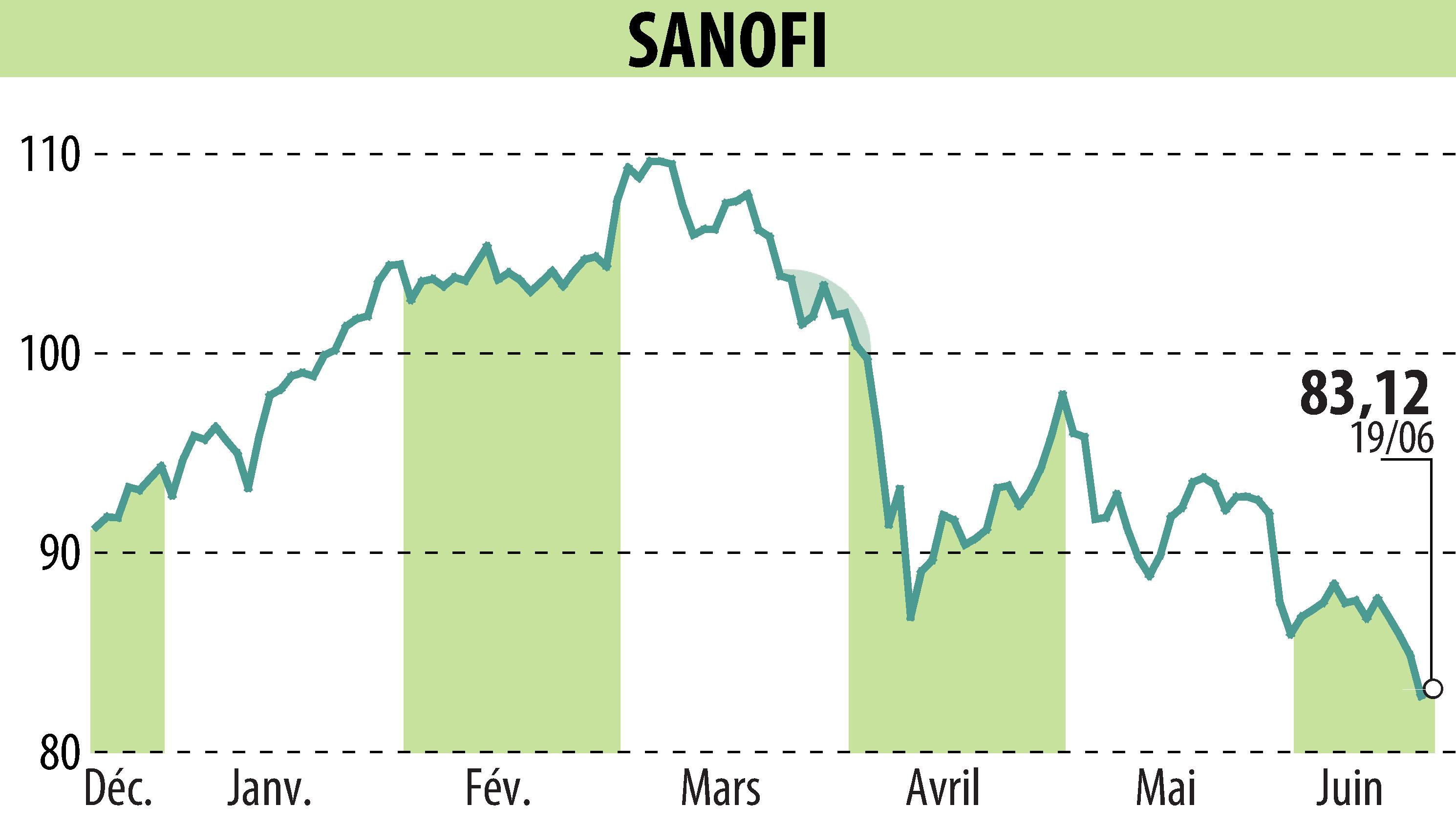
The U.S. Food and Drug Administration (FDA) has approved Dupixent (dupilumab) for treating adult patients with bullous pemphigoid (BP). This condition primarily affects the elderly and is marked by intense itching, painful blisters, and skin lesions. Dupixent becomes the only targeted treatment option for BP in the US, offering hopes for sustained remission and reduced symptoms.
BP, a rare and debilitating skin disease, affects approximately 27,000 adults in the US who do not respond well to systemic corticosteroids. Dupixent's approval relies on pivotal results from the ADEPT phase 2/3 study, which showed significant improvements over placebo. The drug demonstrated reductions in itch, lower steroid usage, and allowed some patients to achieve sustained disease remission.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
