on SANOFI-AVENTIS (EPA:SAN)
Sanofi's Tolebrutinib Regulatory Submission Delayed
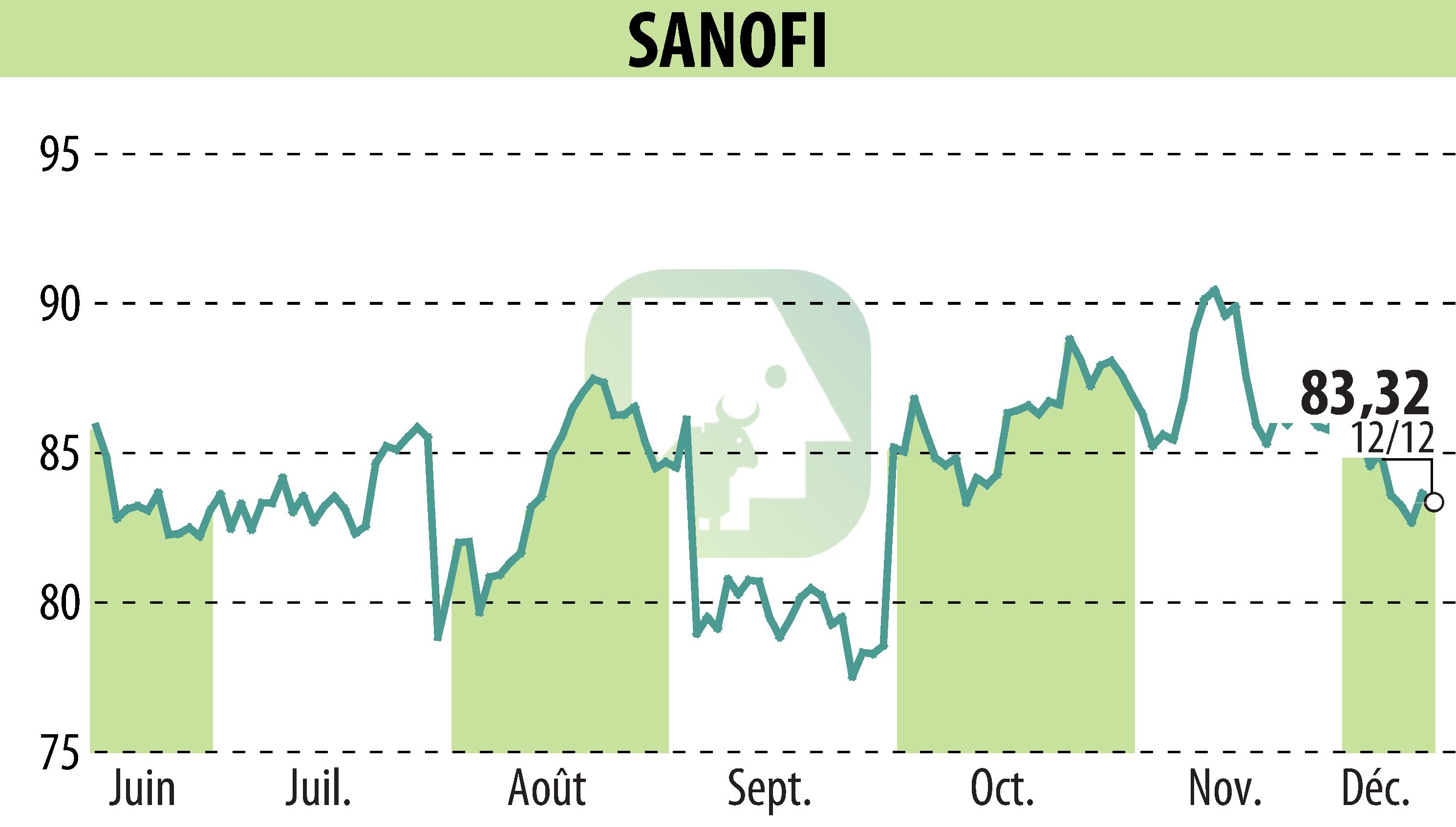
Sanofi has announced a delay in the regulatory review process for tolebrutinib, aimed at treating non-relapsing secondary progressive multiple sclerosis (nrSPMS). Initially expecting a decision from the FDA by December 28, 2025, Sanofi now anticipates further guidance by the end of the first quarter of 2026. This delay stems from ongoing discussions with the FDA.
In response to an FDA request, Sanofi has submitted an expanded access protocol for nrSPMS, demonstrating their commitment to patient access. Despite the delay, Sanofi remains confident in the potential benefits of tolebrutinib, which specifically targets neuroinflammation—a driver of disability progression in multiple sclerosis.
The ongoing regulatory discussions highlight Sanofi's ongoing efforts to address significant unmet medical needs in the treatment of multiple sclerosis and other neurological disorders.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
