on SANOFI-AVENTIS (EPA:SAN)
Sanofi’s Wayrilz Gets FDA Approval as First BTK Inhibitor for ITP
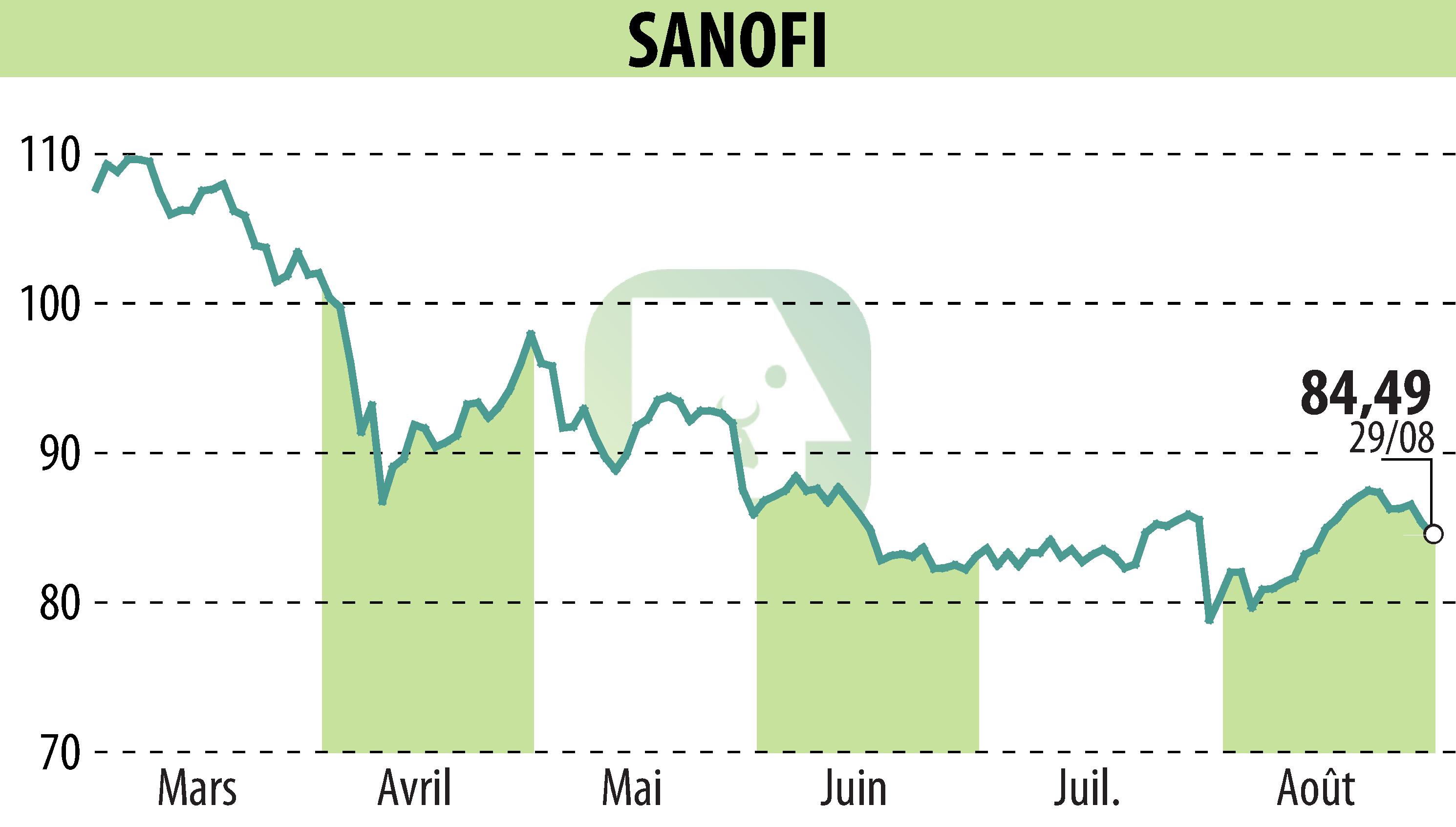
The US FDA has granted approval for Sanofi’s Wayrilz (rilzabrutinib), marking it as the first Bruton’s tyrosine kinase (BTK) inhibitor for adults with immune thrombocytopenia (ITP) who exhibit insufficient responses to prior treatments. This development follows the positive outcomes of the LUNA 3 phase 3 study, which highlighted Wayrilz’s potential in maintaining platelet counts and alleviating other ITP symptoms.
ITP, a disorder known for causing low platelet counts and bleeding, affects over 25,000 adults in the US. The study demonstrated that Wayrilz led to a statistically significant improvement in platelet responses, with patients showing quicker and sustained results compared to those on placebo. The treatment also reported benefits in patients' quality of life scores.
Wayrilz’s approval showcases its novel approach to tackling ITP through multi-immune modulation. Sanofi aims to fulfill unmet patient needs, especially for those who haven't benefitted from existing therapies.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
