on SANOFI-AVENTIS (EPA:SAN)
Sanofi's Wayrilz recommended by CHMP for IPT
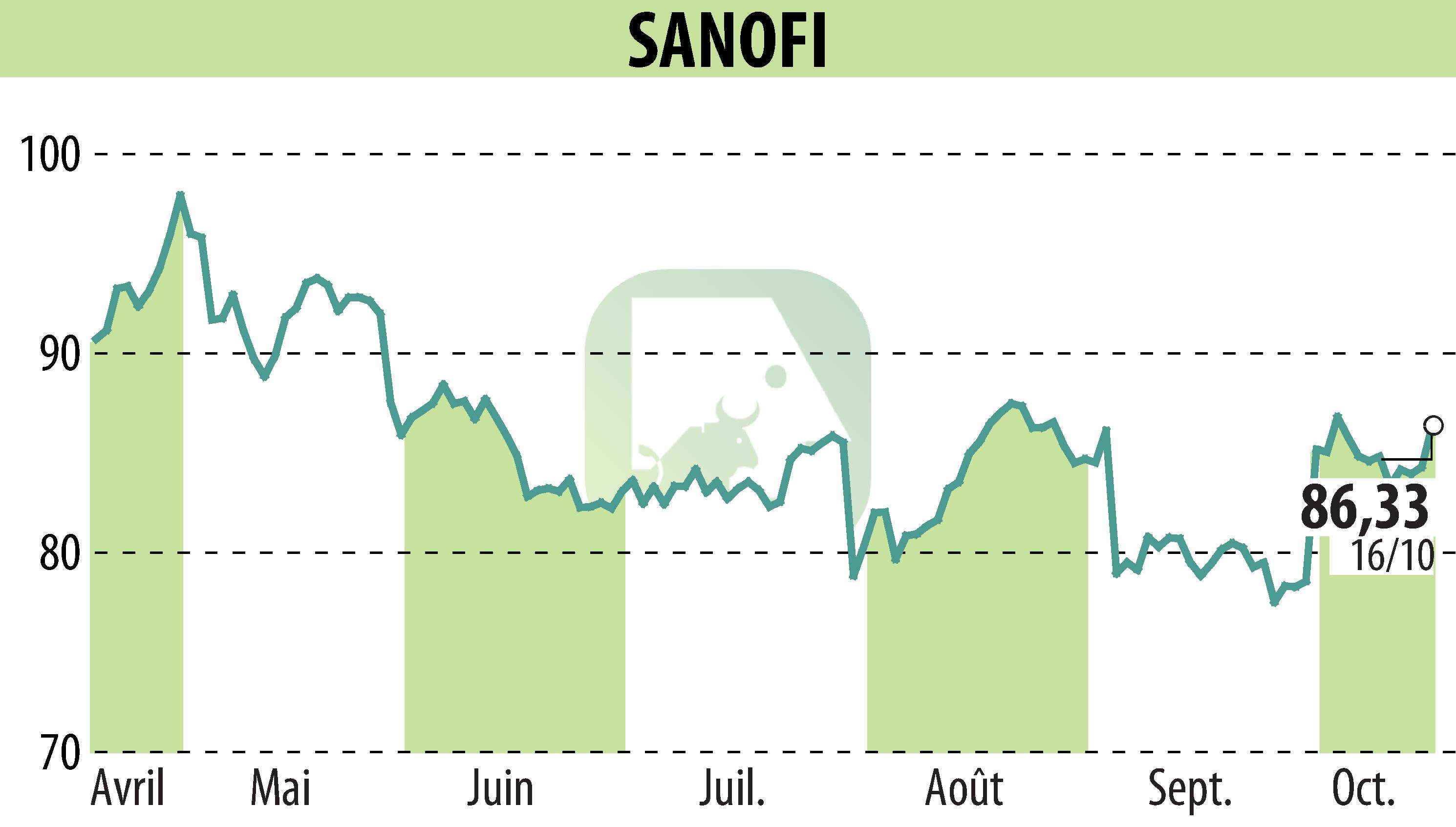
Wayrilz, Sanofi's treatment for immune thrombocytopenia (ITP), has received a positive recommendation from the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP). This decision follows results from the Phase 3 LUNA 3 study, which demonstrated the efficacy of Wayrilz in terms of platelet response and improvement in ITP symptoms.
If approved, Wayrilz will become the first BTK inhibitor to treat IPD in the European Union, targeting the immune dysregulation that causes the disease through multi-immune modulation.
The treatment, already approved in the United States and the United Arab Emirates, is also under review in China. In addition to IPT, Wayrilz is being studied for other rare diseases such as autoimmune hemolytic anemia.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
