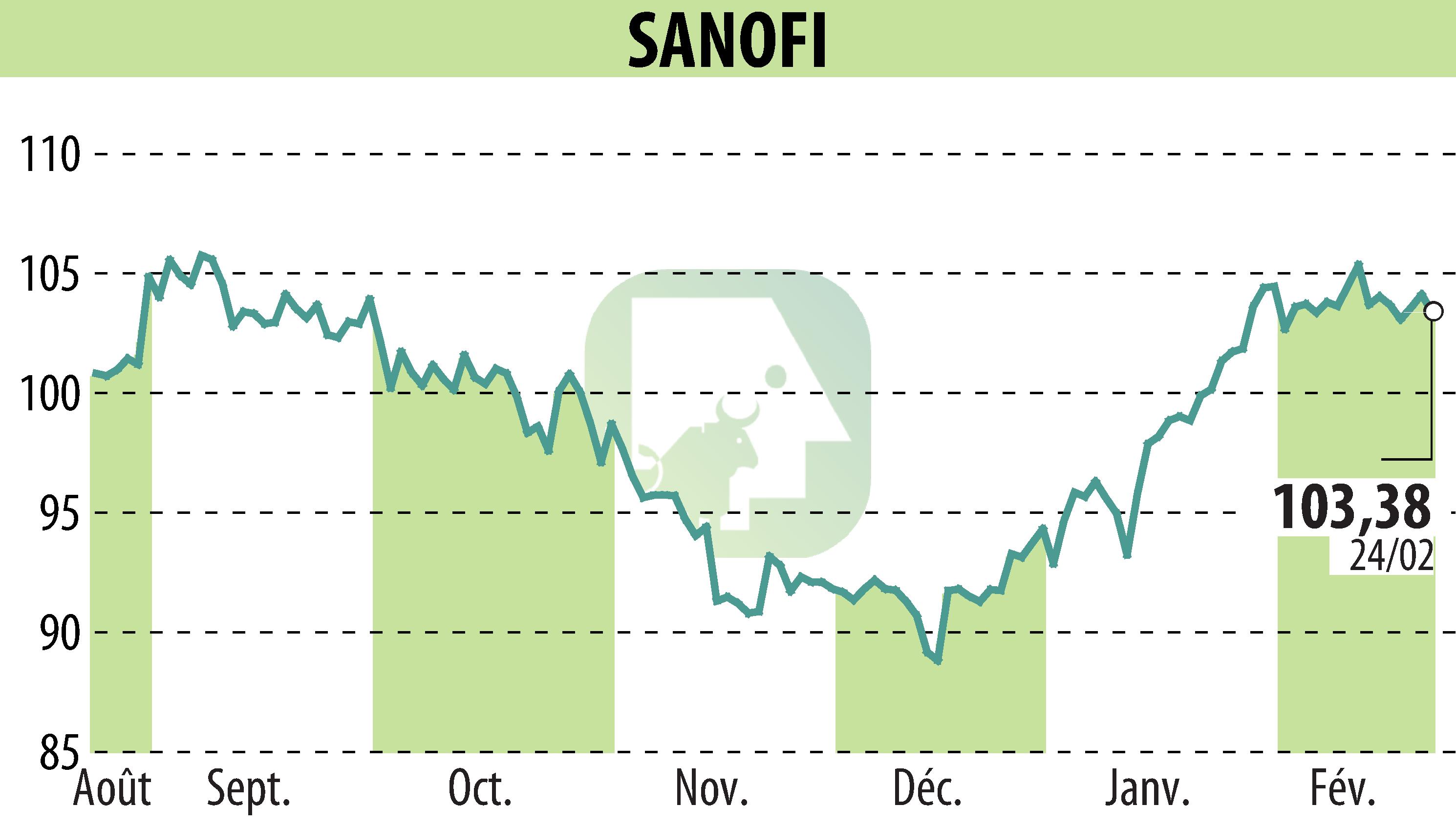
on SANOFI-AVENTIS (EPA:SAN)
Sarclisa Gains Approval in Japan for Multiple Myeloma Treatment
The Japanese Ministry of Health, Labour and Welfare has approved Sarclisa, in combination with VRd, for treating newly diagnosed multiple myeloma patients. This decision is founded on positive results from the IMROZ phase 3 study, which highlighted Sarclisa's significant impact on progression-free survival compared to VRd alone.
Olivier Nataf, Global Head of Oncology at Sanofi, highlighted the growing incidence of multiple myeloma in Japan and the need for novel treatments. This approval marks the first for Sarclisa in newly diagnosed patients in Japan, further extending its reach as a front-line treatment in Japan, the EU, and the US.
Globally, Sarclisa is approved in over 50 countries and is currently under evaluation in several clinical trials, including studies exploring a new subcutaneous administration method. Sanofi emphasizes its ongoing commitment to advancing treatment options for challenging cancers.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
