on SANOFI-AVENTIS (EPA:SAN)
Sanofi obtains approval in China for Qfitlia and Cablivi
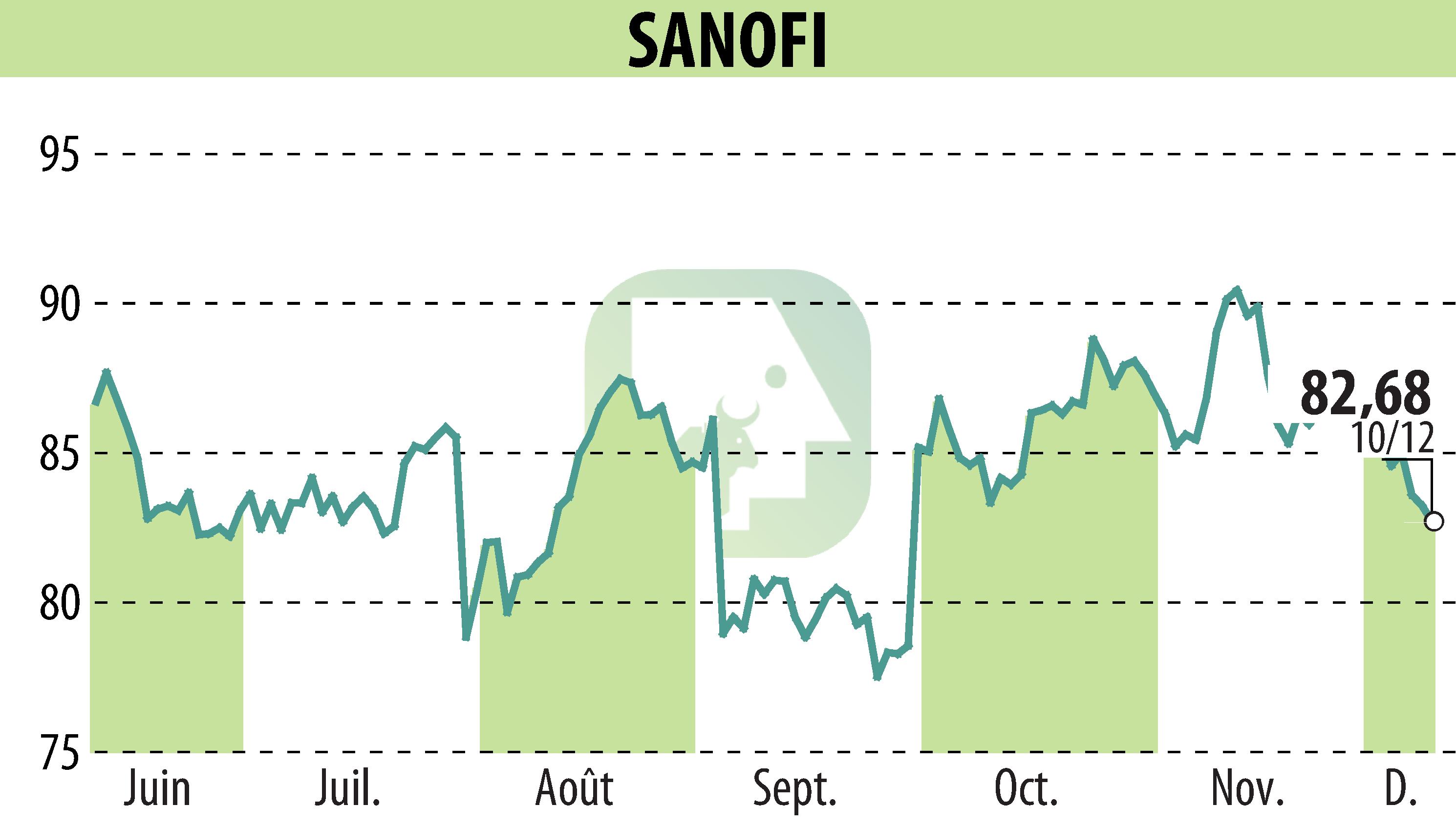
Sanofi has announced the approval of Qfitlia and Cablivi in China by the National Medical Products Administration. These two drugs, intended for rare hematological diseases, reinforce Sanofi's commitment to the Chinese market. Qfitlia, a treatment for hemophilia, is distinguished by its simplified administration: only six injections per year. It has demonstrated its efficacy in the ATLAS studies, significantly reducing bleeding. Cablivi, on the other hand, targets acquired thrombotic thrombocytopenic purpura, a rare and potentially fatal disorder. It works by inhibiting the interaction between von Willebrand factor and platelets, thus reducing the risk of organ damage. These innovations demonstrate Sanofi's commitment to providing medical solutions to critical needs in the field of rare diseases.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
