on SANOFI-AVENTIS (EPA:SAN)
Sanofi's Wayrilz Gets EU Approval for Treating Immune Thrombocytopenia
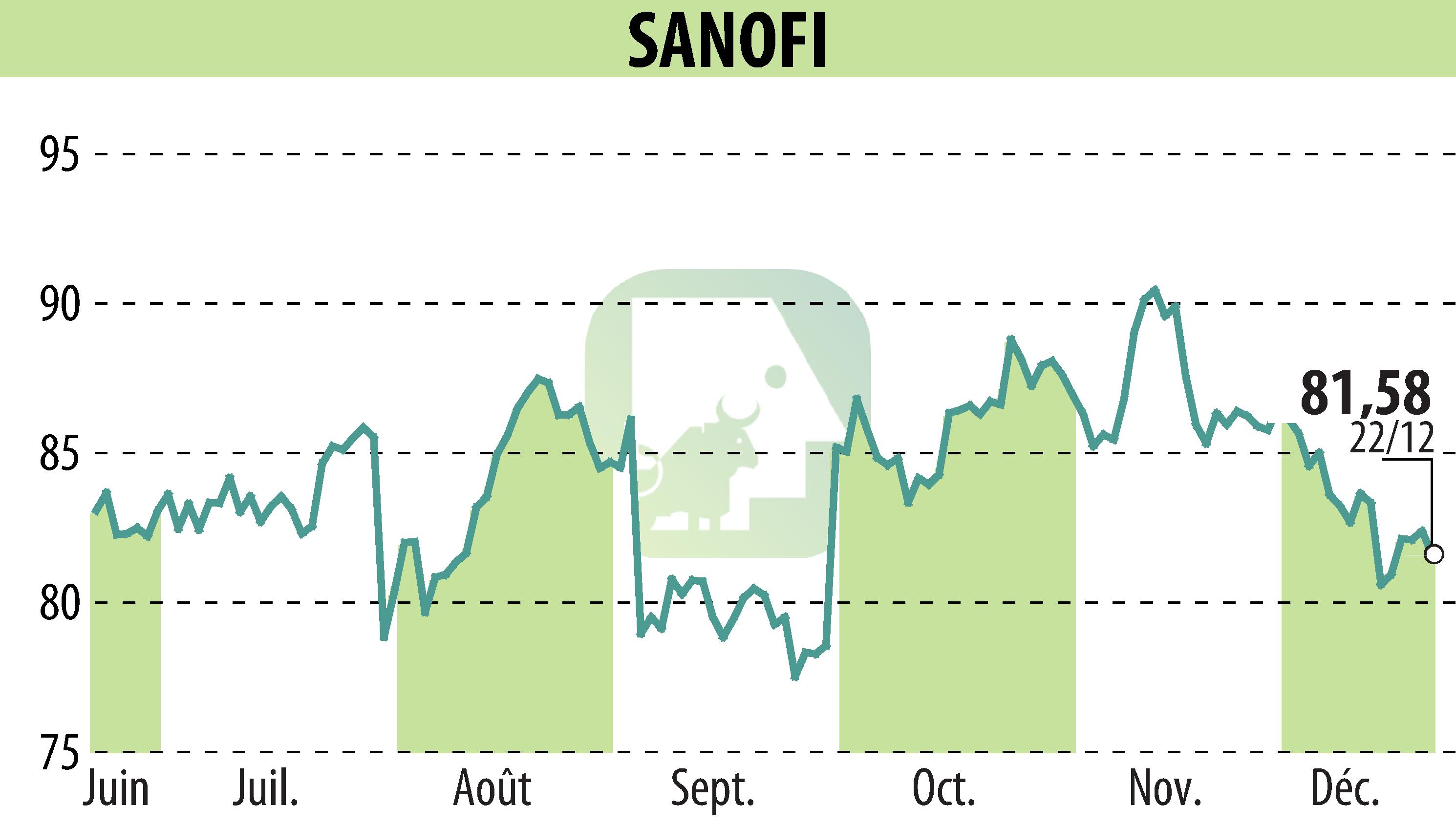
Sanofi has received European Commission approval for Wayrilz (rilzabrutinib), a novel Bruton’s tyrosine kinase (BTK) inhibitor, as a treatment for immune thrombocytopenia (ITP) in adults unresponsive to other treatments. The approval is based on the LUNA 3 phase 3 study, which showed improvement in platelet response and other ITP symptoms. ITP, a rare immune disorder, leads to low platelet counts and impacts quality of life.
Wayrilz uses multi-immune modulation to target various immune system pathways, addressing the disease's underlying causes. The LUNA 3 study reported significant improvements in platelet response and quality of life for patients using Wayrilz.
Already approved in the US and UAE, Wayrilz is under review in Japan and China, with further investigations for other rare diseases. Adverse reactions include diarrhea, nausea, and headache.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
