on MEDINCELL (EPA:MEDCL)
Teva's Olanzapine Injectable Suspension Gains FDA Nod
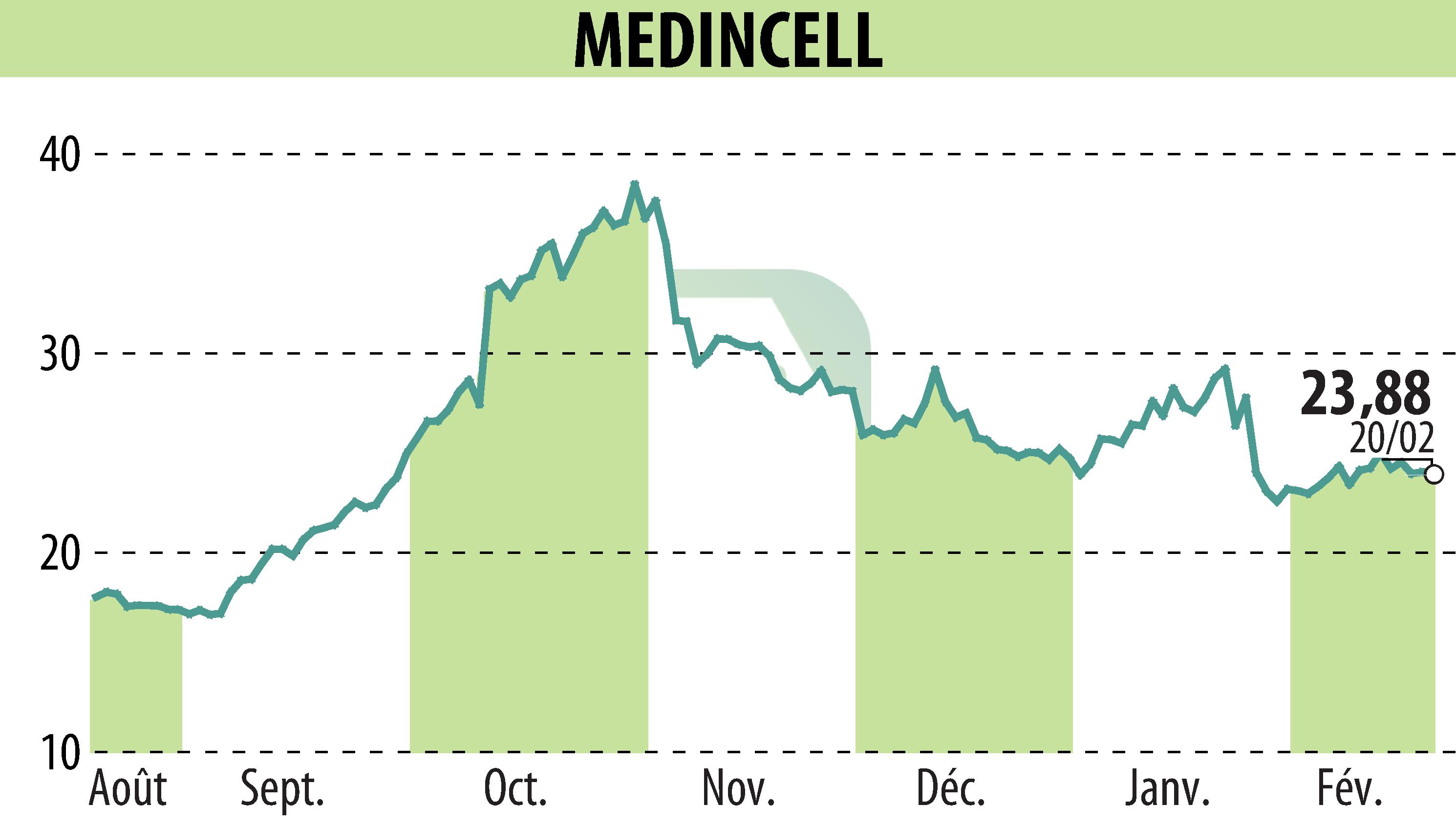
The U.S. FDA has accepted Teva Pharmaceuticals' New Drug Application (NDA) for their olanzapine extended-release injectable suspension, known as TEV-'749. This once-monthly treatment for schizophrenia aims to improve patient adherence and long-term stability.
Currently, no long-acting olanzapine formulation exists without a Risk Evaluation and Mitigation Strategy (REMS). The Phase 3 SOLARIS trial results indicated TEV-'749 showed effectiveness and safety comparable to existing options, potentially eliminating the need for post-injection monitoring.
Teva highlights the unmet need for long-acting olanzapine solutions, especially given that daily oral olanzapine is widely used. Medincell, in collaboration with Teva, uses its proprietary SteadyTeq™ technology for sustained olanzapine release, enhancing treatment effectiveness.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all MEDINCELL news
