on Tharimmune Inc. (NASDAQ:THAR)
Tharimmune Reports Positive Preclinical Results for Oral Antibody TH023
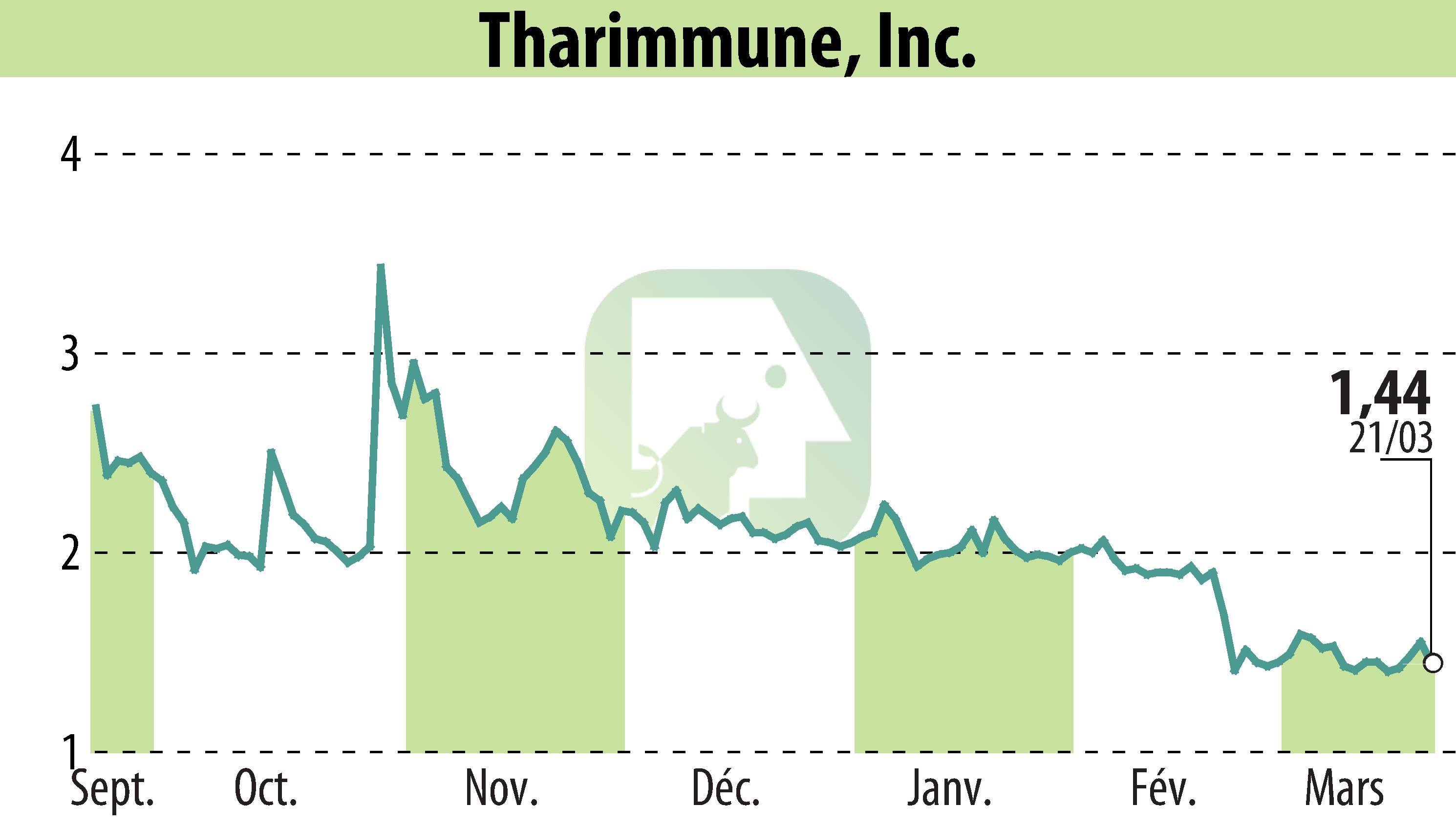
Tharimmune, Inc. announced promising preclinical results for its innovative oral antibody, TH023. The study showed successful delivery of infliximab into serum at concentrations higher than needed for injection-based treatments. Conducted on a murine model, the proprietary protease enzyme stabilization platform enabled efficient serum delivery. This advancement suggests a more convenient alternative to current intravenous or subcutaneous infliximab treatments.
The preclinical evaluation demonstrated TH023's potential to protect infliximab against digestive enzymes, providing effective delivery into local colonic tissue and systemic circulation. This positions TH023 as a promising option for treating gastrointestinal and systemic inflammatory diseases, including inflammatory bowel disease. Tharimmune plans to start clinical trials within the next 12 months.
The study highlighted the ineffectiveness of conventional peptide delivery technologies, emphasizing the flexibility of TH023’s unique formulation. Leveraging a collaboration with Intract Pharma, Tharimmune aims to enhance infliximab's efficacy. This could improve patient compliance and potentially reduce healthcare burdens related to ongoing intravenous therapies, capturing a share of the infliximab market.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Tharimmune Inc. news
