on Tharimmune Inc. (NASDAQ:THAR)
Tharimmune's TH104 Moves Closer to Addressing Weaponized Fentanyl Threat
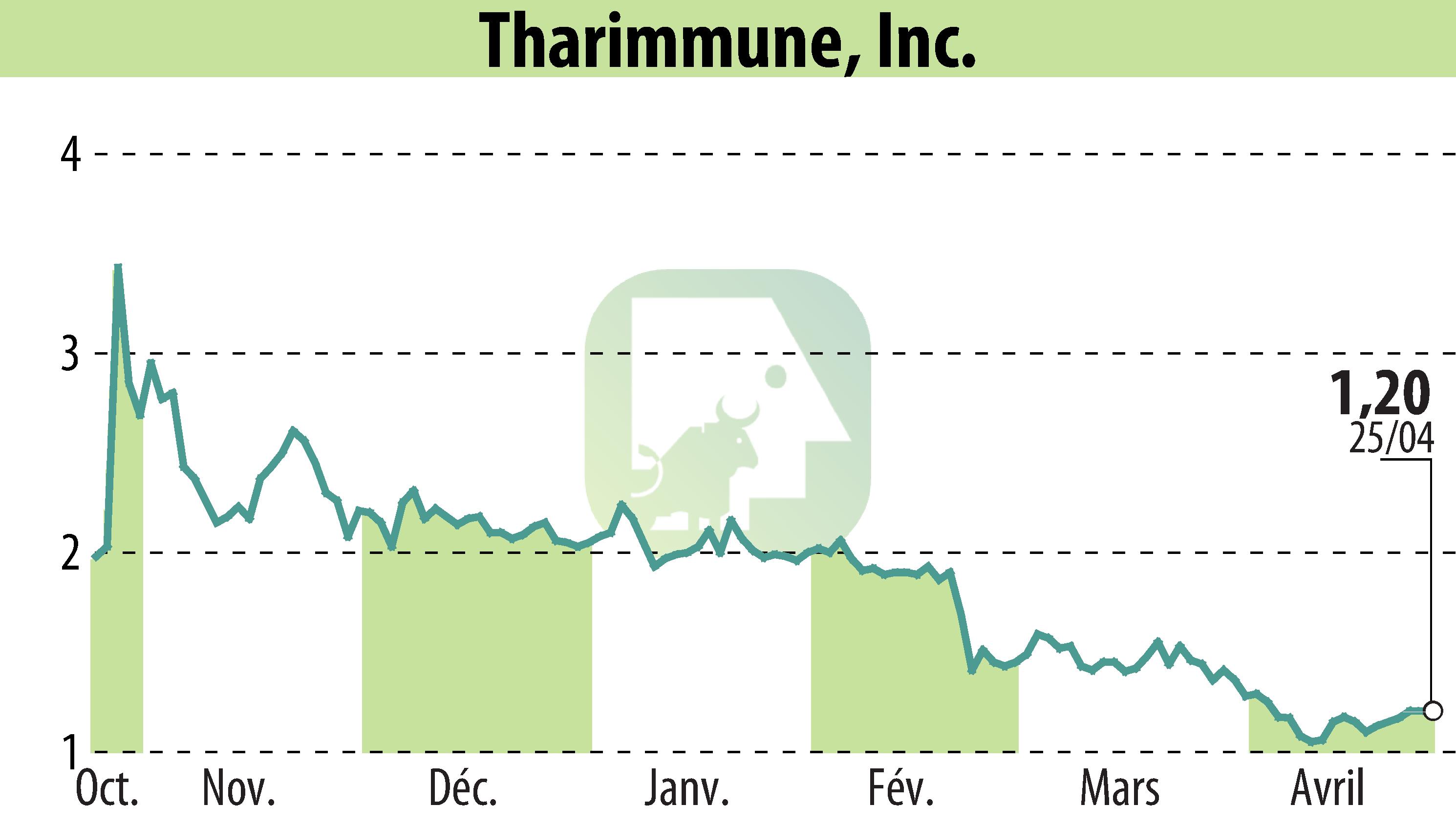
Tharimmune Inc. recently announced positive feedback from the FDA for TH104, a buccal film formulation of nalmefene. The feedback indicates that no additional clinical trials are needed before a 505(b)(2) NDA submission. TH104 is designed for rapid absorption and may bypass liver metabolism, making it suitable for temporary prophylaxis against respiratory and nervous system depression in military personnel and responders to chemical incidents involving high-potency opioids.
This development is critical as it aligns with government efforts to combat the potential weaponization of opioids like fentanyl. The drug offers promise due to its buccal film delivery, which could provide advantages in environments where responders use protective gear. Tharimmune's initiative coincides with increased focus on preparedness by the U.S. government.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Tharimmune Inc. news
