on THERACLION (EPA:ALTHE)
Theraclion Announces 2025 Half-Year Financial Results and Strategic Progress
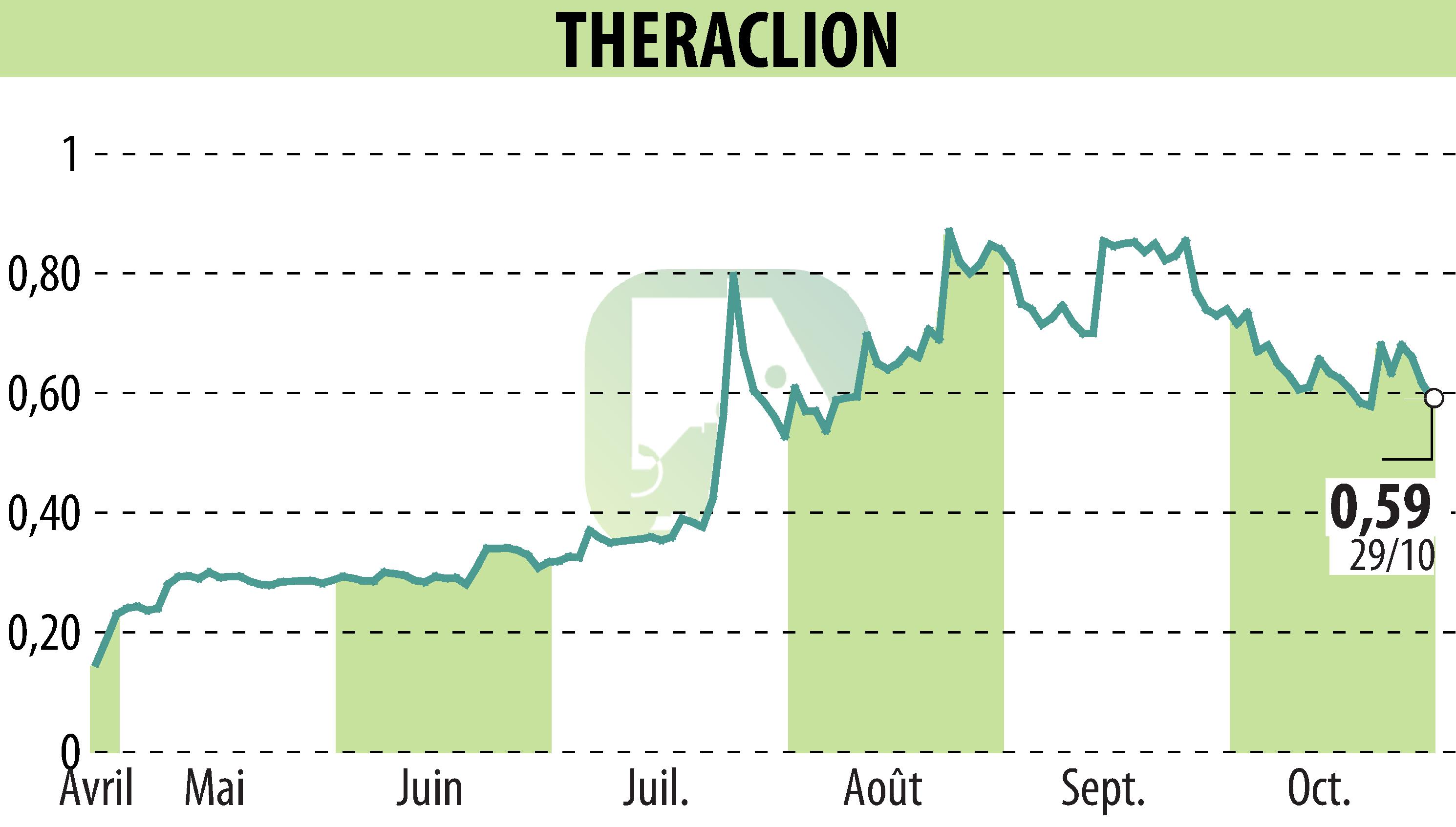
Theraclion, a company known for its robotic platform Sonovein®, reported significant advancements and first-half 2025 financial results. The pivotal study in the U.S. confirmed 96.8% efficacy in treating varicose veins, while Europe and China certifications enhanced market access. Revenue grew by 89%, and operating losses decreased by 23%.
In the U.S., the FDA pivotal study showed promising results, with plans for regulatory submission imminent. European sales saw expansion, particularly in Bulgaria and Spain, aided by CE MDR certification. In China, GB 9706.1-2020 certification marked crucial regulatory progress. The rise in consumable and service revenues underlines Sonovein®’s growing adoption, with commercial launches reinforced by scientific publications and a new brand identity.
Theraclion’s revenue was €834K for the first half of 2025. The firm recorded a 17% net loss reduction. Its financial health was supported by convertible bond issues and shareholder commitments, providing a stable cash flow for continued development.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all THERACLION news
