on THERACLION (EPA:ALTHE)
Theraclion Submits Sonovein to FDA After Year of Key Achievements
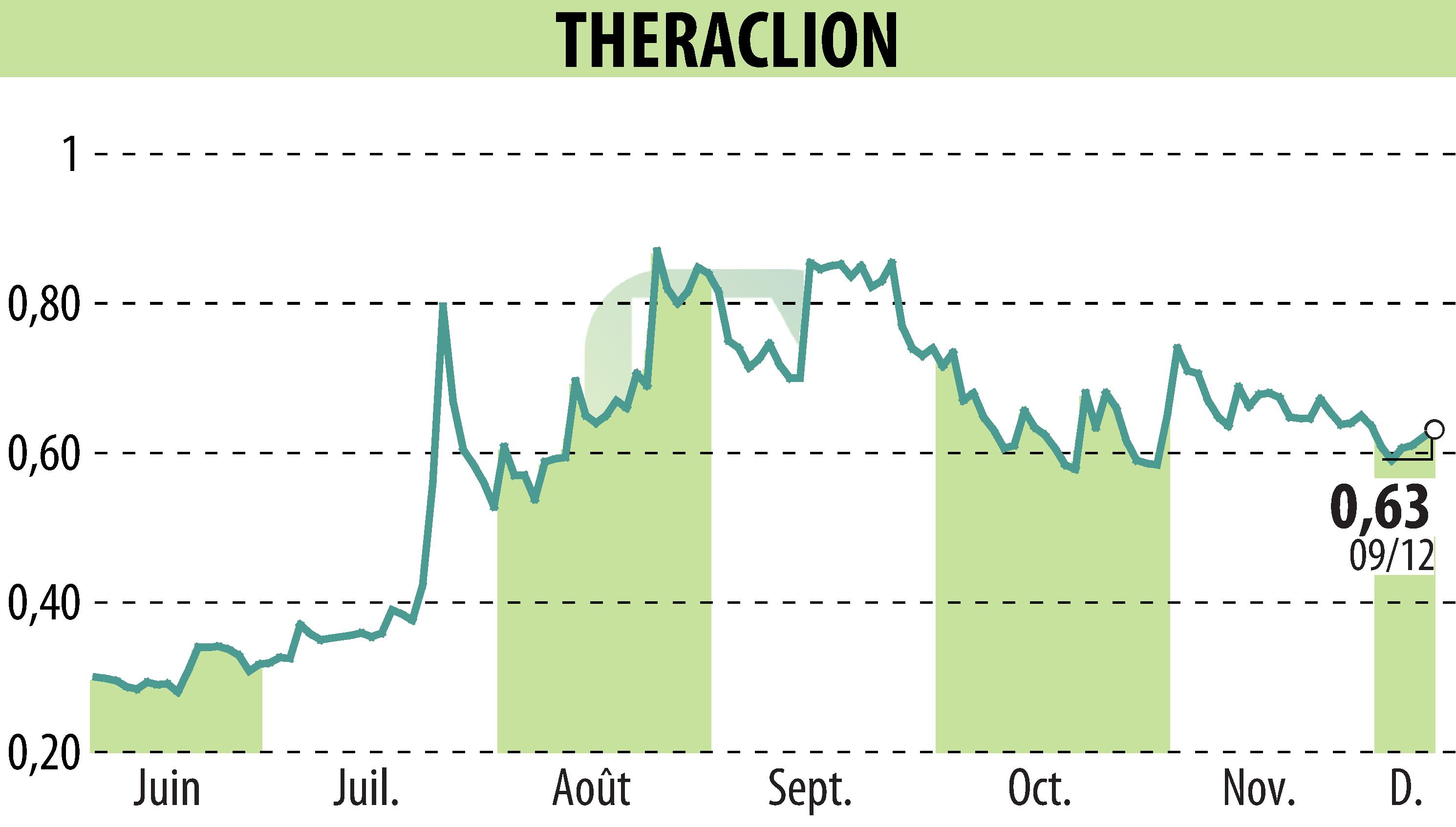
Theraclion, a company focusing on non-invasive varicose vein treatments, has announced the submission of its Sonovein® platform to the FDA. This pivotal move marks the culmination of a year filled with significant regulatory and clinical progress. The data packet submission is crucial for De Novo clearance, potentially opening doors to the extensive U.S. market by mid-2026.
Beyond the U.S., Sonovein® has gained important regulatory approvals in Europe and China, achieving EU MDR certification and compliance with China's standards. This progress reinforces its potential for future commercialization in these regions.
Clinical evidence reinforcing Sonovein®'s efficacy was strengthened through various peer-reviewed studies in 2025, with impressive results in terms of vein occlusion and patient safety. The year also saw active participation in 16 international congresses, enhancing visibility among medical professionals globally.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all THERACLION news
