on UCB (EBR:UCB)
UCB Reports Positive Phase 3 Trial Results for Fenfluramine in CDKL5 Disorder
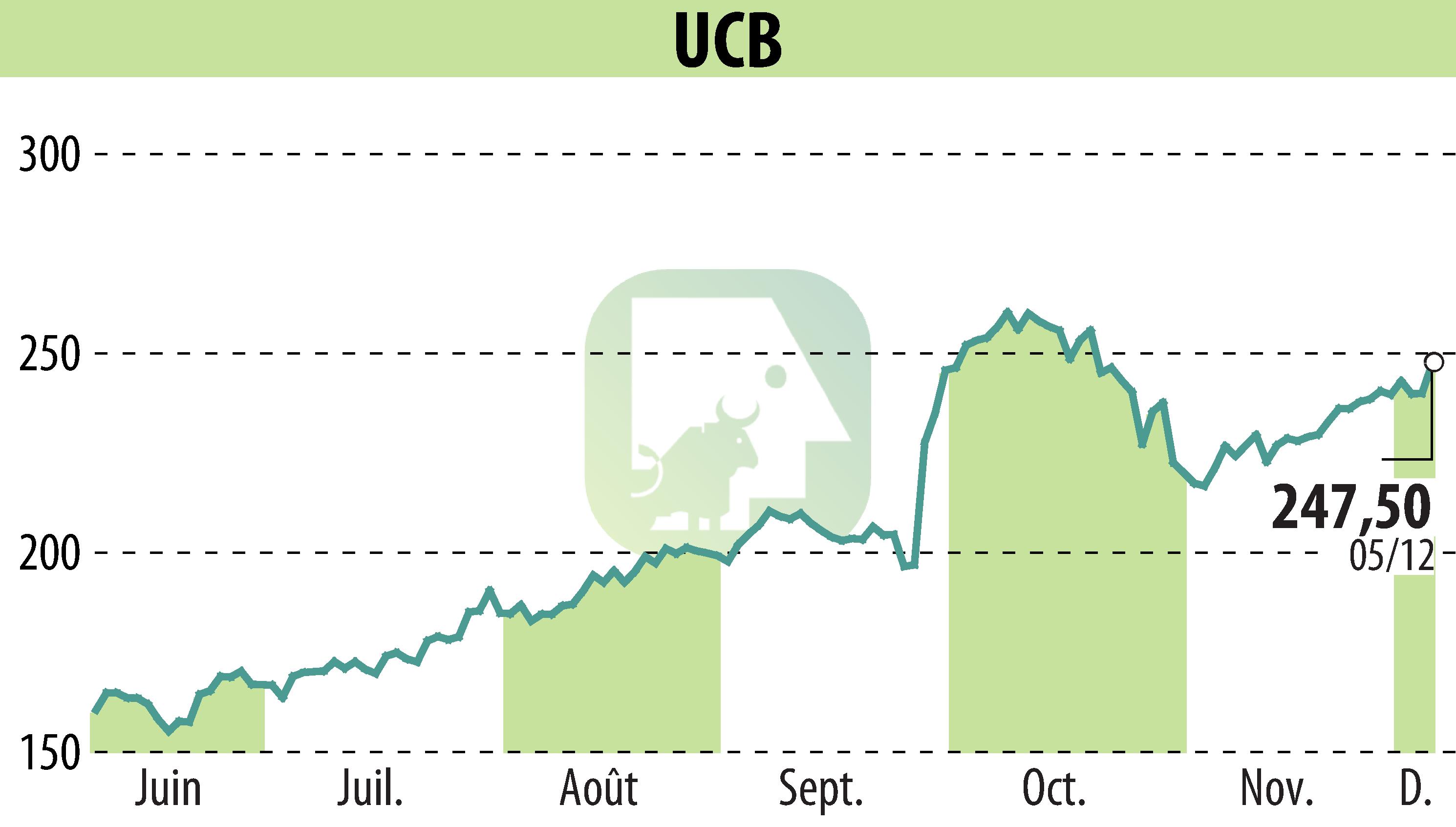
UCB, a biopharmaceutical company, presented encouraging results from its GEMZ phase 3 study at the American Epilepsy Society meeting. The study assessed the efficacy and safety of fenfluramine in reducing countable motor seizure frequency in patients with CDKL5 Deficiency Disorder (CDD). The trial met its primary endpoint, showing a significant reduction in seizure frequency compared to placebo.
Secondary outcomes highlighted meaningful improvements in global impressions of patient conditions. Fenfluramine was well tolerated with no new safety concerns identified. UCB intends to seek regulatory approval for fenfluramine in treating CDD seizures, marking its potential third approval in developmental and epileptic encephalopathy.
The phase 3 study included 86 participants aged 1–35, demonstrating notable reductions in seizure frequency and improvement in patient conditions. Ongoing studies aim to evaluate long-term safety and tolerability.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all UCB news
