on AMOEBA (EPA:ALMIB)
Unanimous green light for Amoéba's active substance in Europe
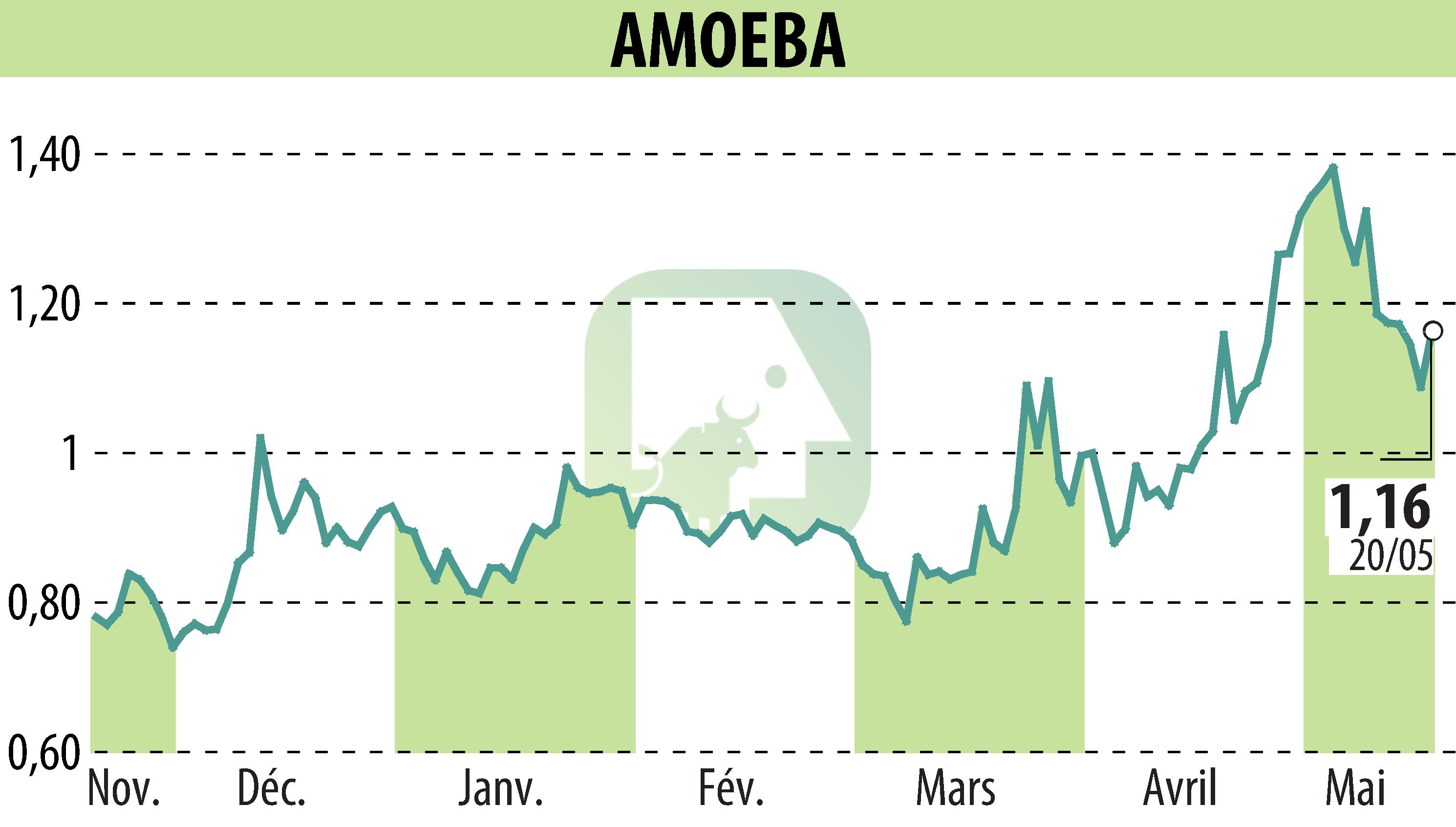
Amoéba, a green biotechnology specialist based in Chassieu, announced that all 27 European Union member states have unanimously approved its Willaertia magna C2c Maky lysate. This active biocontrol substance would be included in AXPERA, which aims to combat diseases such as grapevine downy mildew. This unanimous support facilitates the product's commercialization in Europe as early as 2026.
The European Food Safety Authority had already published a positive report on this substance in January 2025. This unanimous vote paves the way for official approval by the European Commission.
Amoéba's CEO, Jean-François Doucet, is delighted with this key milestone. The process for obtaining marketing authorization in several European countries is underway.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all AMOEBA news
