on VALNEVA (EPA:VLA)
Valneva Announces Positive Phase 2 Results for Chikungunya Vaccine in Children
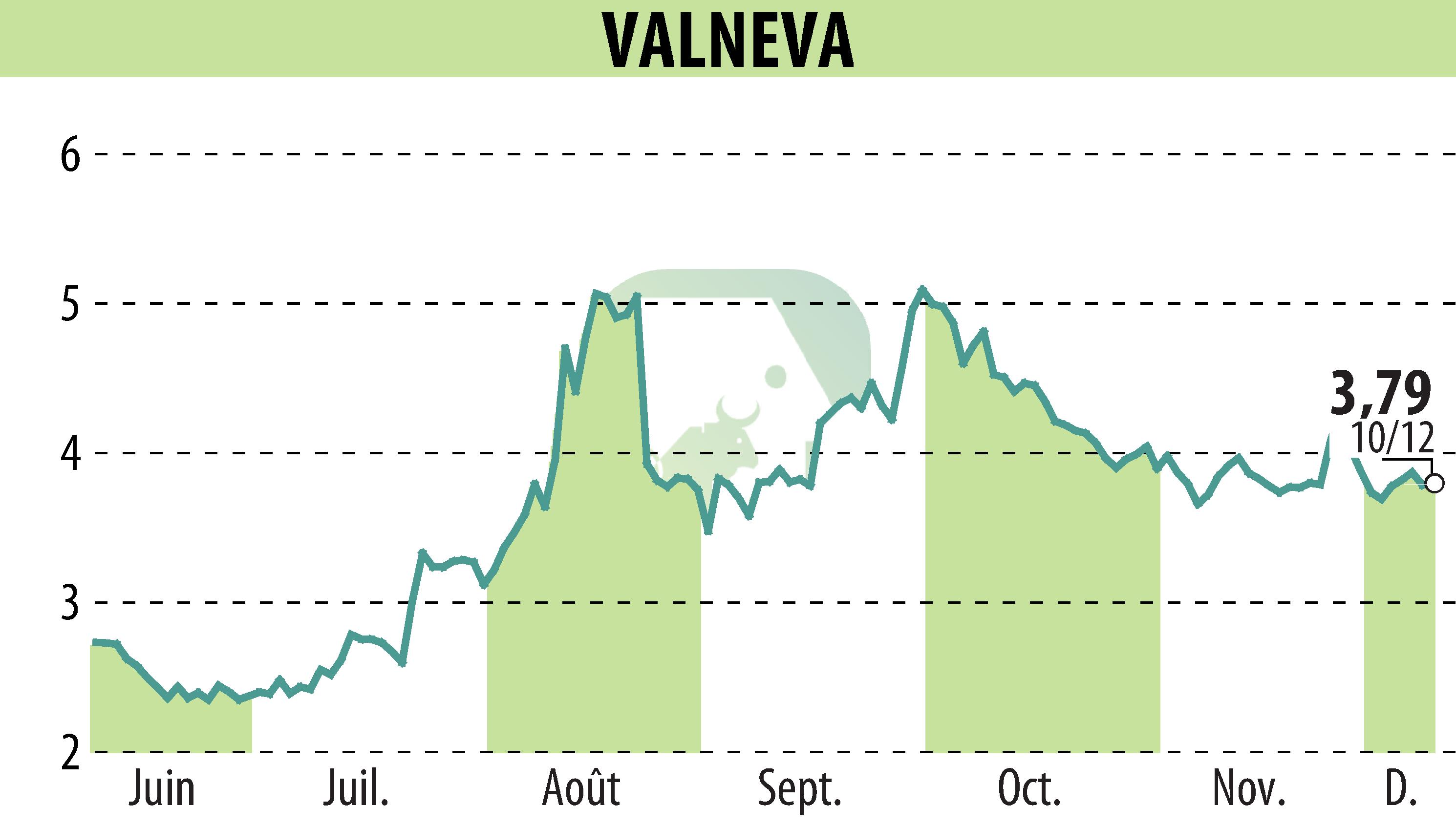
Valneva SE has released positive final results from its Phase 2 trial of the chikungunya vaccine, IXCHIQ®, in children aged one to eleven. The vaccine demonstrated strong antibody persistence and safety. The trial included 304 children and showed that the full dose elicited a more robust immune response 12 months post-vaccination compared to the half dose. Notably, the vaccine was well tolerated, irrespective of prior chikungunya infection.
This study, partially funded by CEPI and supported by the European Union, provides a basis for selecting the full dose for forthcoming Phase 3 trials. The continued investigation in adolescent populations will aid in refining the approach for Phase 3 trials in children.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all VALNEVA news
