on VALNEVA (EPA:VLA)
Valneva Obtains FDA Lift of Ban on IXCHIQ®
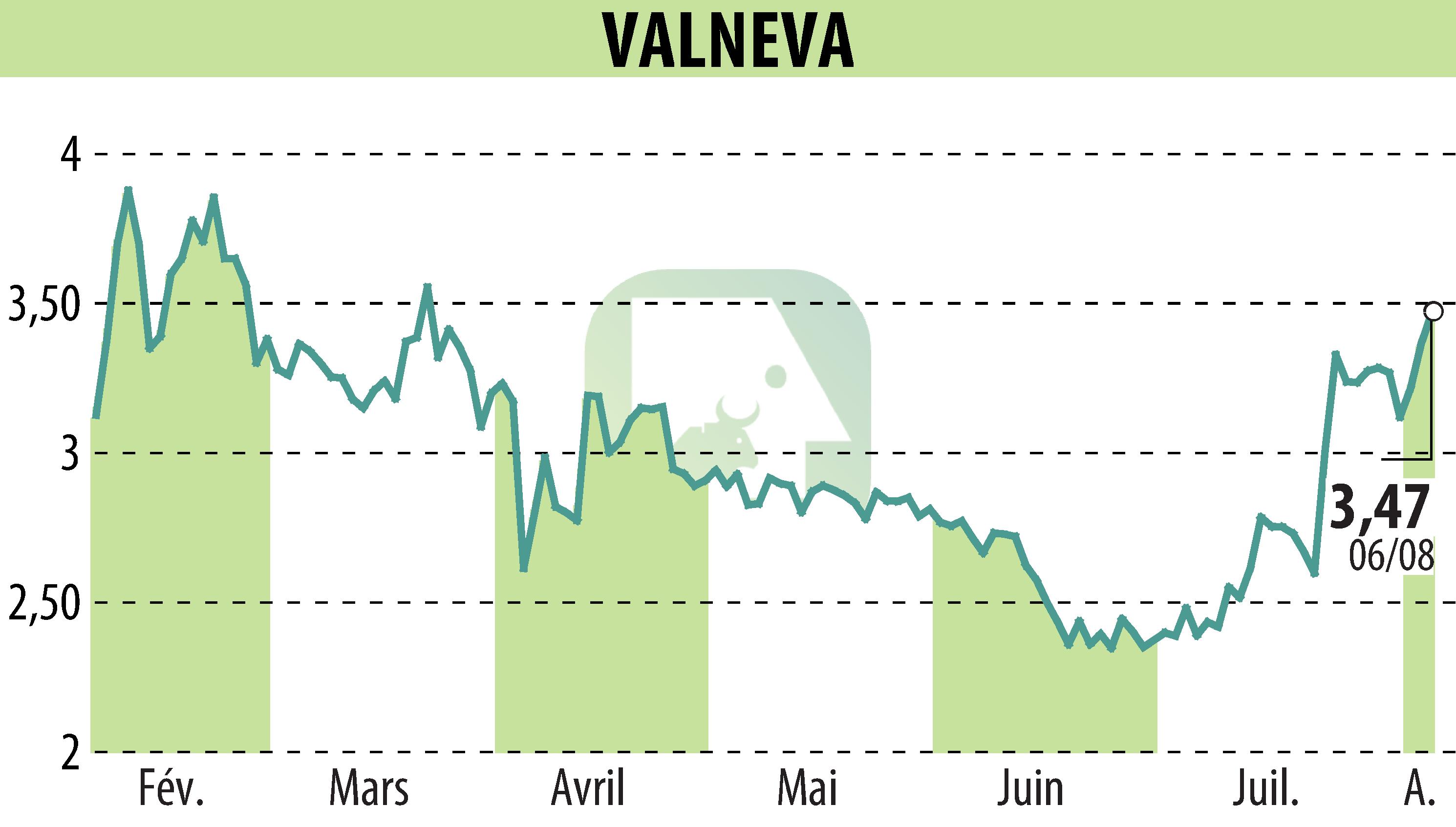
On August 7, 2025, Valneva SE announced that the FDA has lifted the temporary hold on the use of IXCHIQ® chikungunya vaccine in individuals aged 60 years and older. This follows a similar decision by the EMA in July. IXCHIQ® remains indicated for the prevention of chikungunya in the United States in high-risk adults.
The updated product specifications address serious adverse events observed in older adults with chronic conditions. The FDA emphasizes that the vaccine is not necessary for most U.S. travelers and should be administered cautiously after evaluating the risks and benefits.
Revisions are underway to expand the potential indication of the vaccine to adolescents and include data on antibody persistence.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all VALNEVA news
