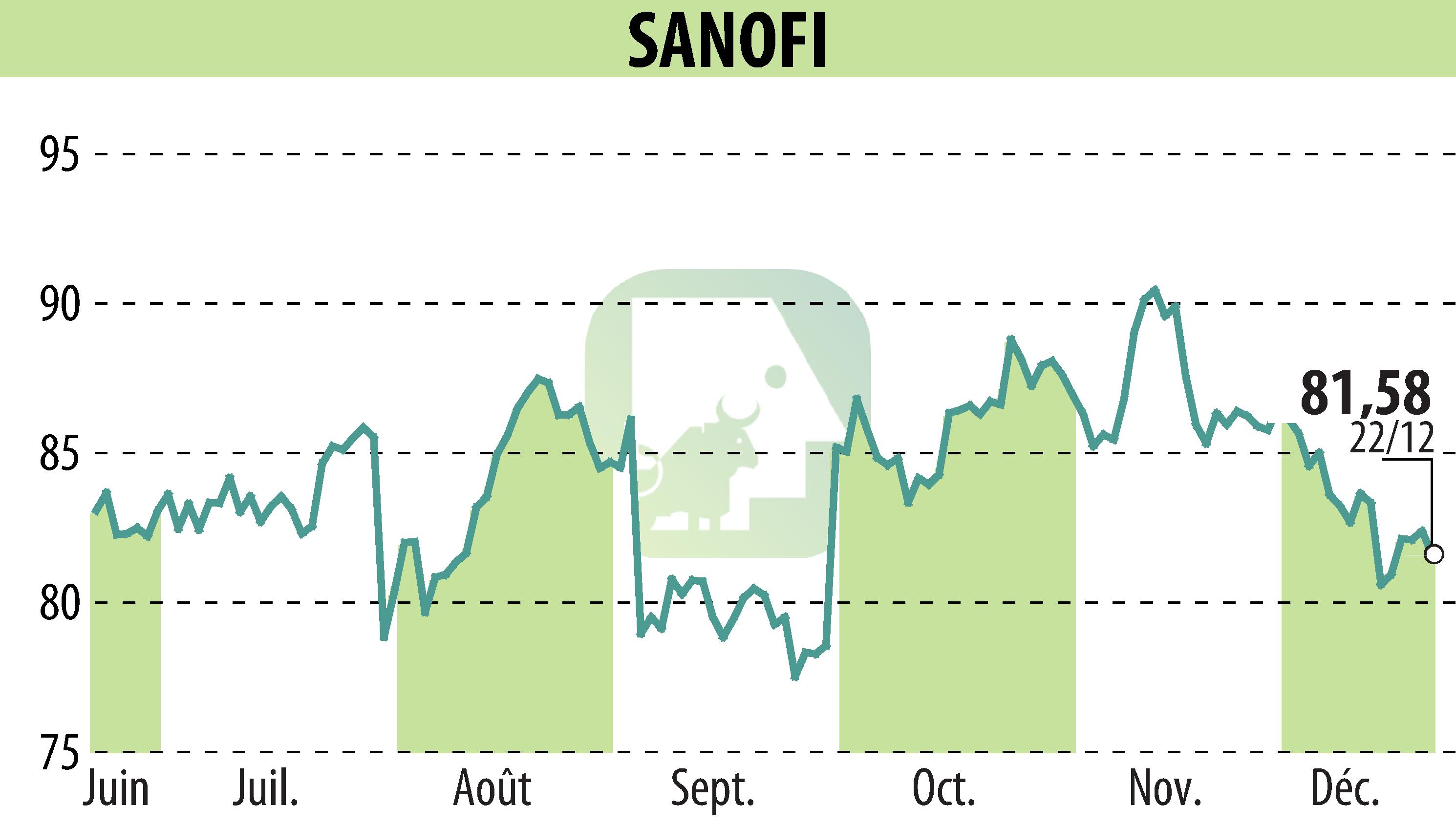
on SANOFI-AVENTIS (EPA:SAN)
Sanofi and Regeneron's Dupixent approved in Japan for children with asthma
The Japanese Ministry of Health has approved Dupixent for the treatment of bronchial asthma in children aged 6 to 11. This drug, developed by Sanofi and Regeneron, is already used for older patients. The approval is based on phase 3 studies demonstrating a significant reduction in exacerbations and an improvement in lung function. The observed side effects were primarily injection site reactions and a few cases of eosinophilia. This decision could transform the treatment of severe asthma, which significantly impacts the daily lives of young patients. Dupixent is already approved for several other indications.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
